Preparation method of spermacoce latifolia triterpenoids and application of spermacoce latifolia triterpenoids in preparation of glycosidase inhibitor drug
The technology of a glycosidase inhibitor and a compound is applied to the preparation of the triterpenoid compound of A. chinensis and its application field in the preparation of a glycosidase inhibitor medicine, and achieves the effects of easy storage, abundant sources and high safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
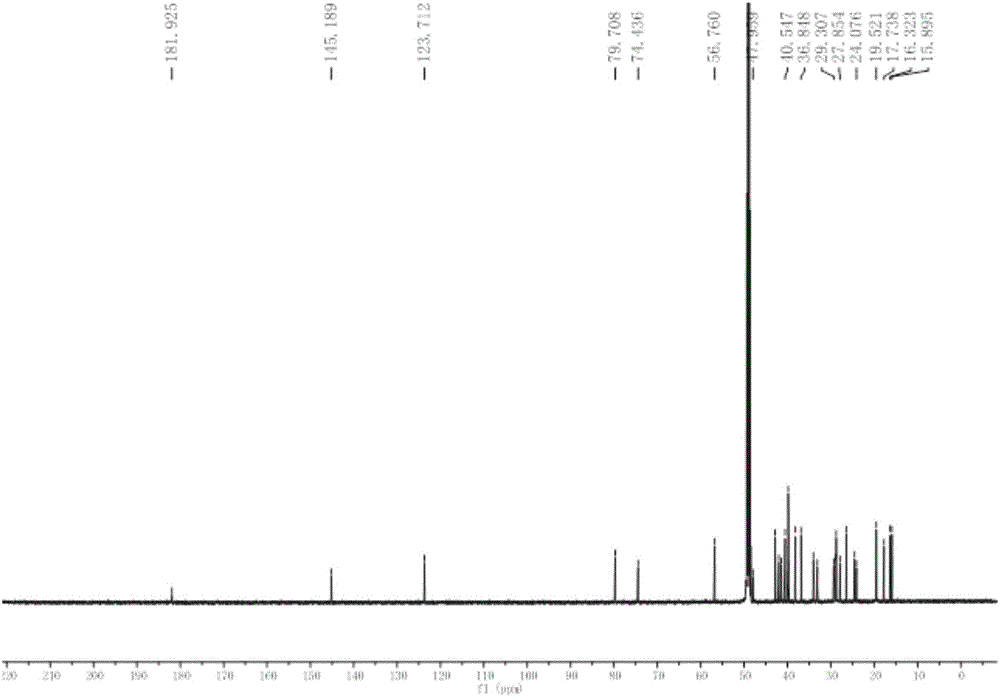
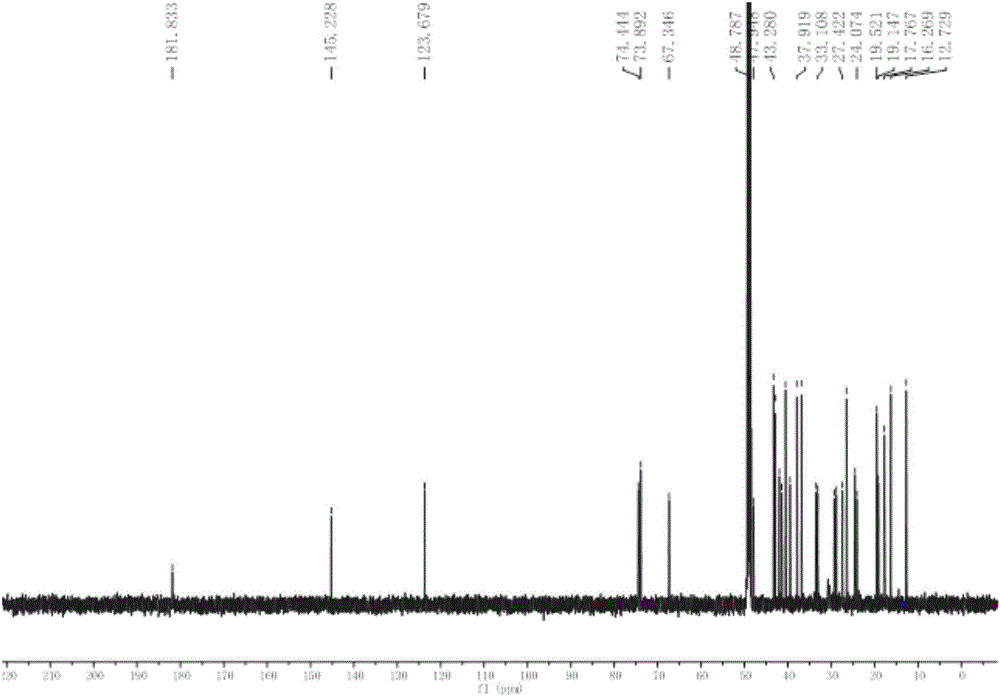
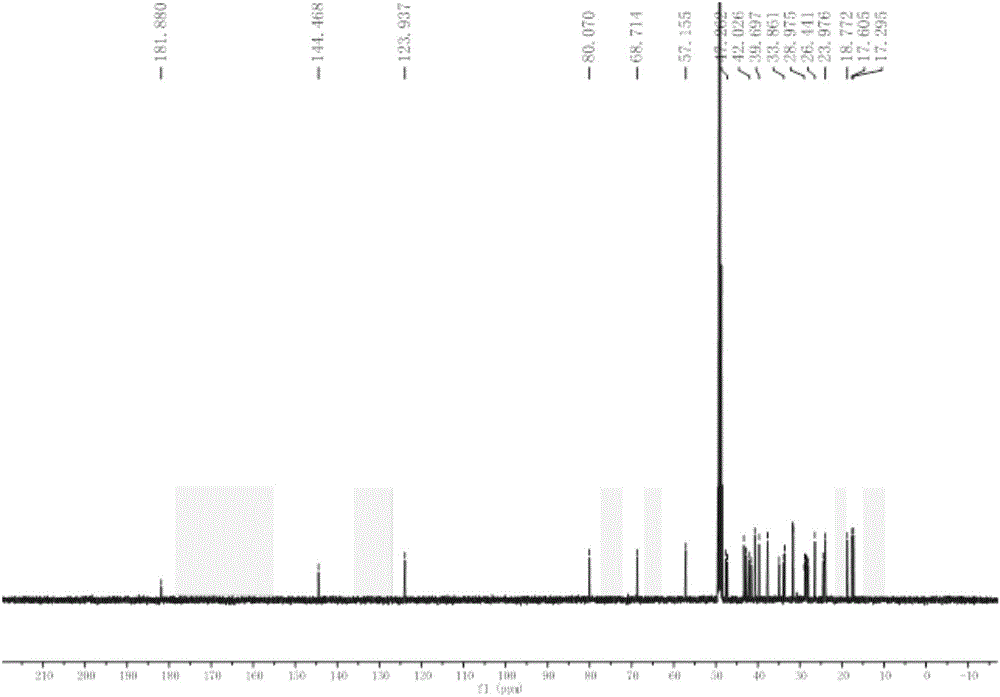
[0028] Embodiment 1: Preparation of six kinds of triterpenoids in broad-leaved abundant flowers and plants
[0029] 1.1 Plant source and identification
[0030] The whole plant of Spermacoce latifolia Aubl.K.Schum. was collected from South China Botanical Garden, Guangzhou City, Guangdong Province in September 2012, and was identified by Xing Fuwu, a researcher of South China Botanical Garden, Chinese Academy of Sciences.
[0031] 1.2 Extraction and separation
[0032] The whole plant (5.0Kg) of the dried Herba chinensis (5.0Kg) was crushed and extracted at room temperature with 95% ethanol aqueous solution, the extracts were combined and concentrated under reduced pressure to obtain an alcohol-free extract. The extract was suspended in water, and extracted four times with petroleum ether and ethyl acetate in turn; after concentration under reduced pressure, petroleum ether extract (330 g) and ethyl acetate extract (202 g) were obtained. The obtained petroleum ether extract ...
Embodiment 2
[0040] Embodiment 2: α-glucosidase inhibitory activity detection of described six kinds of triterpenoids
[0041] 2.1 Instruments and reagents
[0042] Experimental instrument: Genois microplate reader (Tecan GENios, Switzerland).
[0043]Reagent samples: α-glucosidase was purchased from Sigma Chemical Co. (Sigma-Aldrich, St.Louis, USA); Acarbose was purchased from Tokyo Chemical Industry Co., Ltd. (Japan); Phenol-α-D-glucopyranoside (PNPG) was purchased from Tokyo Chemical Industry Co., Ltd. (Japan); six triterpenoid mesembryanth emoidigenic acid (compound 1), 29-hydroxyhederagenin (compound 2), 3β ,6β-dihydroxy-olean-12-en e-28-oic acid (compound 3), scutellaric acid (compound 4), arjunic acid (compound 5), 3β,6β,23-tr ihydroxy-olean-12-en- 28-oic acid (compound 6) is prepared by the method of Experimental Example 1 above, and can also be prepared according to the literature Magina et al., Quim Nova, 2012, 35:1184-1188; Huu et al., J Chem, 2007, 45: 363-367; Ku o et al., ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com