Blocking agent kit for immunochromatography determination and using method thereof
A technology of immune chromatography and blocking agents, which is applied to measuring devices, analytical materials, instruments, etc., can solve the problems of insufficient blocking effect of blocking agents and the inability to completely prevent the interference of endogenous factors, and achieve a good blocking effect , the effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
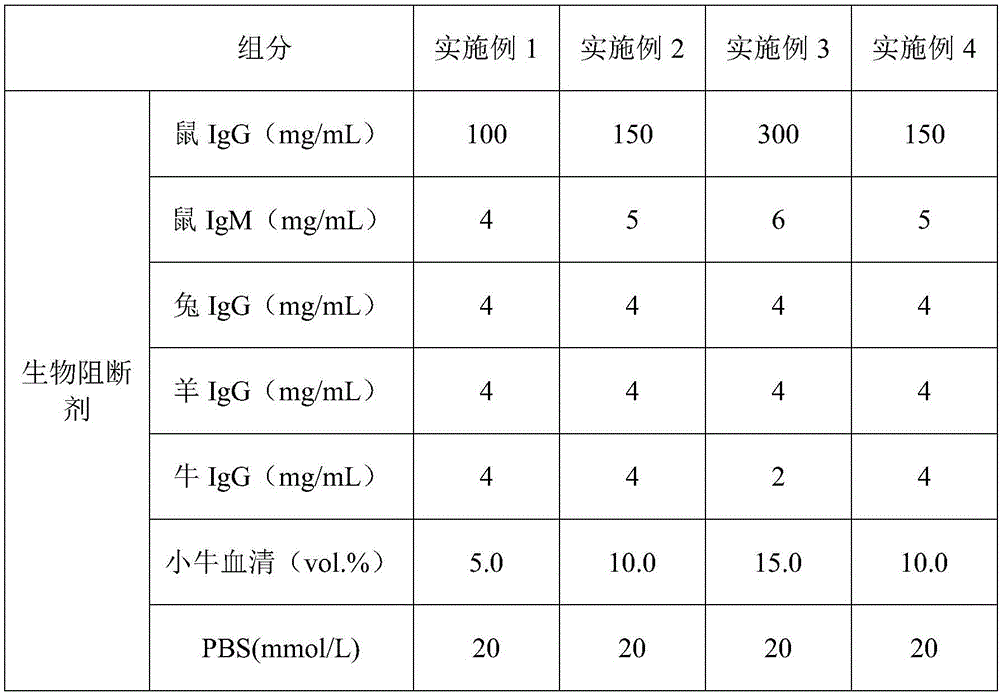
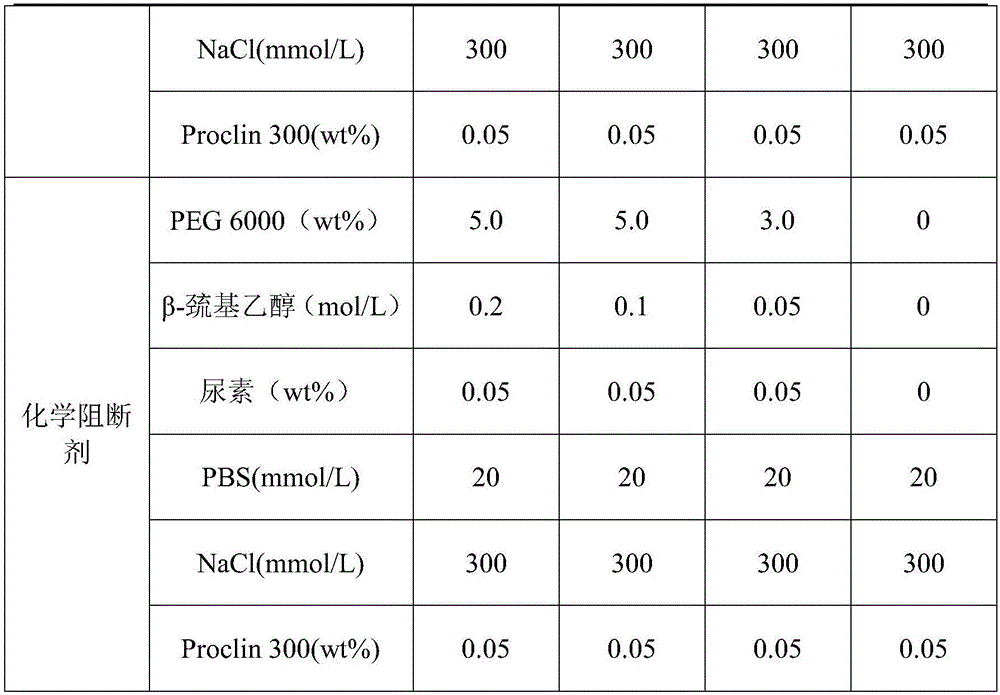
[0034] A blocking agent kit for immunochromatographic determination, comprising a box body and a blocking agent arranged in the box body, the blocking agent includes a biological blocking agent and a chemical blocking agent, and the biological blocking agent It is set in the box body separately from the chemical blocking agent. Wherein the contents of each component in the biological blocking agent and the chemical blocking agent are shown in Table 1.
[0035] The concentration table of each component of compound blocking agent in the embodiment 1-4 of table 1
[0036]
[0037]
Embodiment 5-7
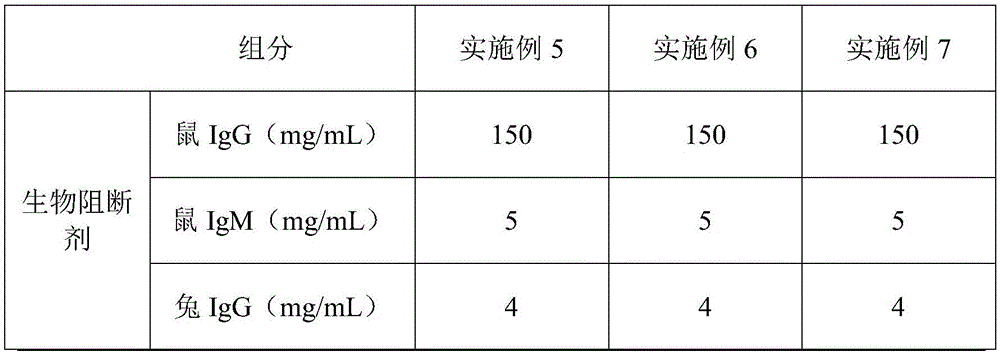
[0039] A blocking agent kit for immunochromatographic determination, a blocking agent kit for immunochromatographic determination, comprising a box body and a blocking agent arranged in the box body, the blocking agent includes biological The blocking agent and the chemical blocking agent, the biological blocking agent and the chemical blocking agent are separately arranged in the box body. The contents of each component in the biological blocking agent and the chemical blocking agent are shown in Table 2.
[0040] Concentration table of each component of composite blocker in table 2 embodiment 5-7
[0041]
[0042]
experiment example 1
[0043] Experimental Example 1: Quantitative determination of cardiac troponin I (cTnI) by fluorescence immunochromatography
[0044]8 cases of human serum samples (experimental samples, specimen number 1-8) confirmed to have obvious endogenous interference and 2 cases of human serum samples confirmed to have no obvious endogenous interference (control samples, specimen number 9-10) were selected as the research Object, prepare sample pad treatment solution and sample diluent according to the method recorded in "Establishment of Colloidal Gold Immunochromatography for Rapid Detection of Avian Influenza Virus and Serum Antibody" (Feng Juan, Yangzhou University, 2006), and then take Example 1 -4 Composite blocking agent prepared by adding the biological blocking agent to the sample pad treatment solution at a ratio of 0.5vol%, and adding the chemical blocking agent to the sample diluent at a ratio of 0.5vol%, and then according to YY / T1221 -The method stipulated in 2013 (cardiac ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com