Di- and triphosphate prodrugs
A technology of compounds and medicinal salts, applied in the field of diphosphate and triphosphate prodrugs, to avoid side effects and improve the effect of actual use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
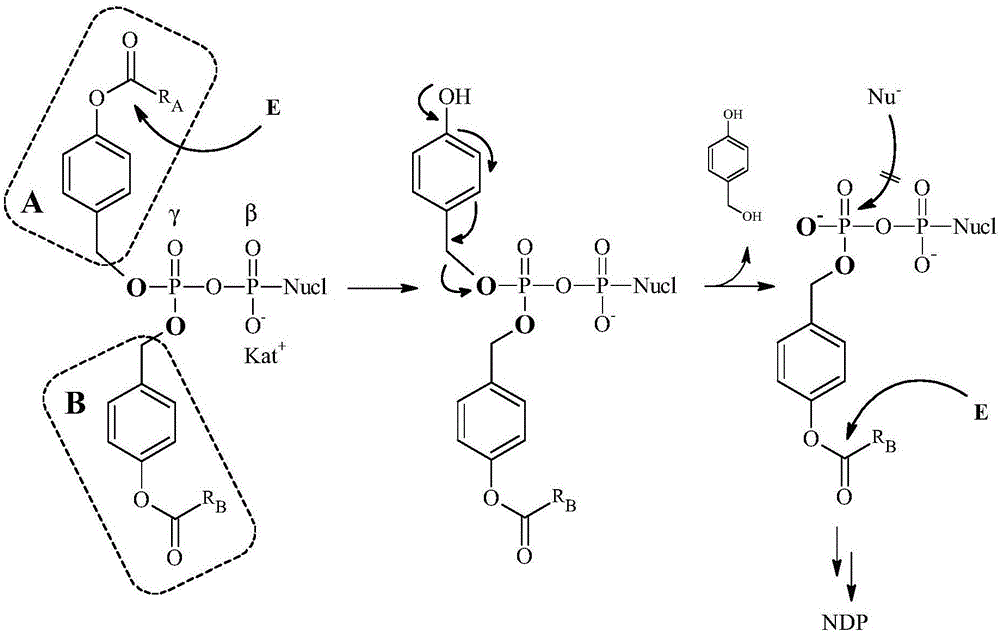
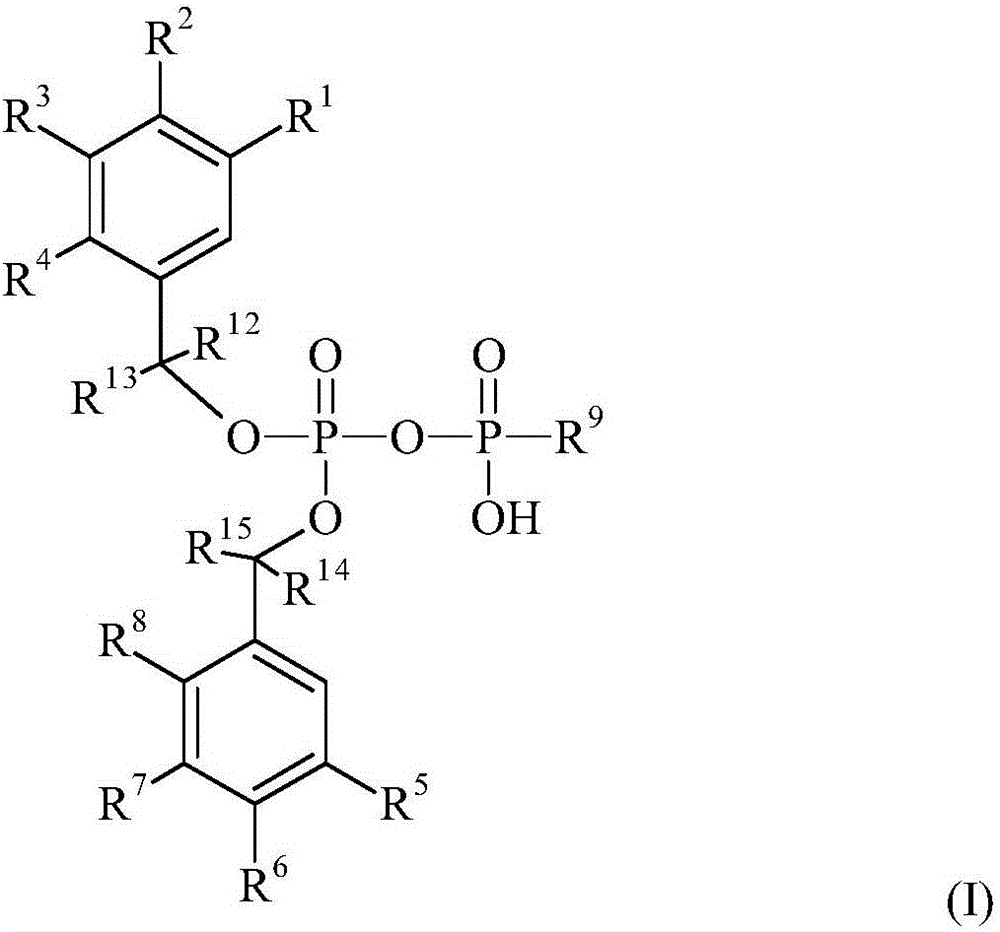
[0127] A schematic diagram of an example of a process for the preparation of compound I of the invention is shown below. The first benzyl alcohol derivative II A with phosphorus trichloride (PCl 3 ) to prepare compound 100, which reacts with N,N-diisopropylamine (NH(iPr) 2 ) react to form the corresponding phosphoramidite compound III. Compound III and the second benzyl alcohol derivative II B The reaction takes place to synthesize the phosphoramidite compound IV. It can then be combined with a mono- or diphosphate compound V, such as nucleoside monophosphate or nucleoside monophosphate analogs (both here abbreviated as NMP), or nucleoside diphosphate or nucleoside diphosphate analogs (both here Abbreviated as NDP) coupling, thereby obtaining compound I. The NDP or NTP compounds of the present invention (including nucleoside analogs containing compounds) may also be referred to herein as DiPPro nucleotides or TriPPPro nucleotides for short.
[0128]
[0129]For the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com