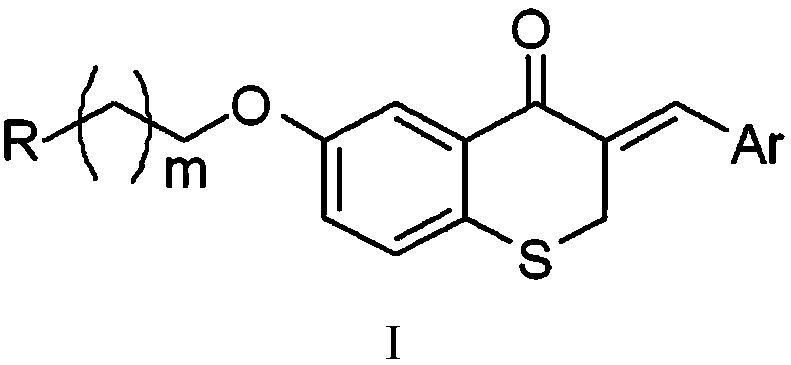
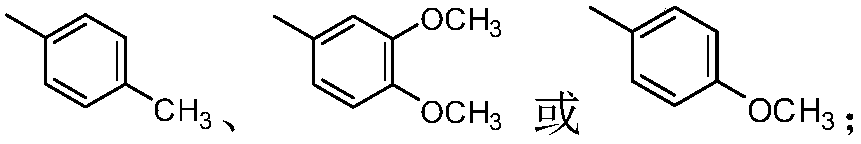
A kind of thiochromanone derivative and its preparation method and application
A technology of thiochromanone and its derivatives, which is applied in the field of thiochromanone derivatives and their preparation, can solve the problems of no discovery and achieve the effects of high specificity, expanding drug selection, and alleviating drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
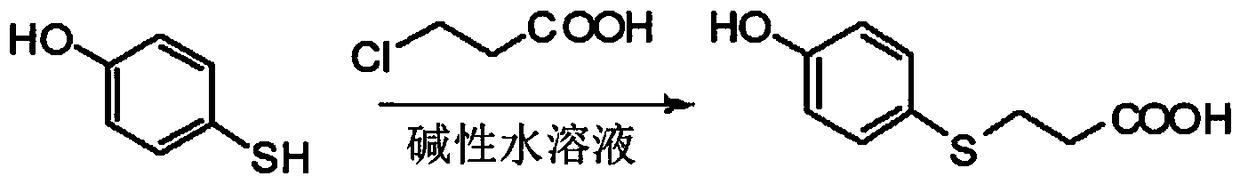
[0043] (1) Dissolve 0.2mol of p-hydroxythiophenol and 0.24mol of β-chloropropionic acid in 30mL of alkaline aqueous solution (KOH aqueous solution with a pH value of 12), react at 80°C for 2.5h, follow the reaction until complete by TLC, Cool to room temperature, adjust the pH value of the reaction solution to 2 with concentrated hydrochloric acid (mass ratio concentration is 36%), separate out a large amount of white precipitates, let stand overnight, filter with suction, wash the filter cake with water, wash with 50mL ethanol aqueous solution (V 乙醇 :V 水 =1:1) recrystallization, vacuum drying overnight at 40°C to obtain 3-p-hydroxyphenyl-3-thiopropionic acid; the chemical reaction process is as follows:
[0044]
[0045] (2) Dissolve 10 g of 3-p-hydroxyphenyl-3-thiopropionic acid in 40 mL of concentrated sulfuric acid (mass ratio concentration is 98%), place it at room temperature for ice thawing, a large amount of solids are precipitated, and use 20 mL of ethanol after su...
Embodiment 2
[0062] Step (1)-(4) is the same as embodiment 1, Where m is 2, step (5) is: oxidizing 1mmol of 3-benzyl-6-(3-bromopropoxy)thiochroman-4-one, 1.2mmol of benzylamine, and 1.5mmol of hydrogen Potassium was dissolved in 8 mL of 1,4-dioxane solvent, reacted at 90°C for 12h, TLC followed the reaction, and stopped the reaction when the product point no longer changed; after the reactant was cooled to room temperature, the solvent was distilled off under reduced pressure, and the obtained Silica gel column for solid (V 乙酸乙酯 :V 丙酮 =4: 1) purification, promptly obtains compound 26 in table 1; Its productive rate is 50%, finds that when the reaction condition of the (5) step of embodiment 1 is 90 ℃ of reaction 9-12 hours in the course of research , Compound 26, which was originally a by-product, became the main product.
[0063] The chemical reaction formula of (5) step is:
[0064]
[0065] The compounds prepared by the methods of Example 1 and Example 2 and their physical and c...
Embodiment 3
[0076] Embodiment 3 The antifungal activity detection test of the compound prepared by the present invention
[0077] In order to explore the in vitro antifungal activity of the synthesized compounds, this experiment respectively measured Candida albicans (Canidia albicans, C.a), Cryptococcus neoformans (Cryptococcus neoformans, C.n), Aspergillus niger (Aspergillus niger, A.n) Mucorus racemosa ( Mucor racemosus, M.r), gypsum-like Microsporum (Microsporum gypseum, M.g) and flocculent Epidermophyton (Epidermophyton floccosum, E.f) and other six kinds of fungi antibacterial activity. The tested fungi were all from the Culture Collection Center of the Chinese Academy of Medical Sciences.
[0078] This experiment adopts the microdilution method to carry out antifungal activity test to 6 kinds of tested fungi, 8 mg of 46 target compounds prepared in Example 1 are first dissolved with 625 μL of dimethyl sulfoxide (DMSO), and 50 μL of this solution is incubated with 2.5 mL of 1640 so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com