Freeze-drying technology of injection lornoxicam
A technology for lornoxicam and injection, which is applied in the field of freeze-drying technology of lornoxicam for injection, can solve the problems of increasing the impurity content of the finished product and long time, and achieve the goal of saving time, saving energy and accelerating the sublimation rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: A kind of lyophilization process of lornoxicam for injection
[0041] Described freeze-drying process comprises the following steps successively:
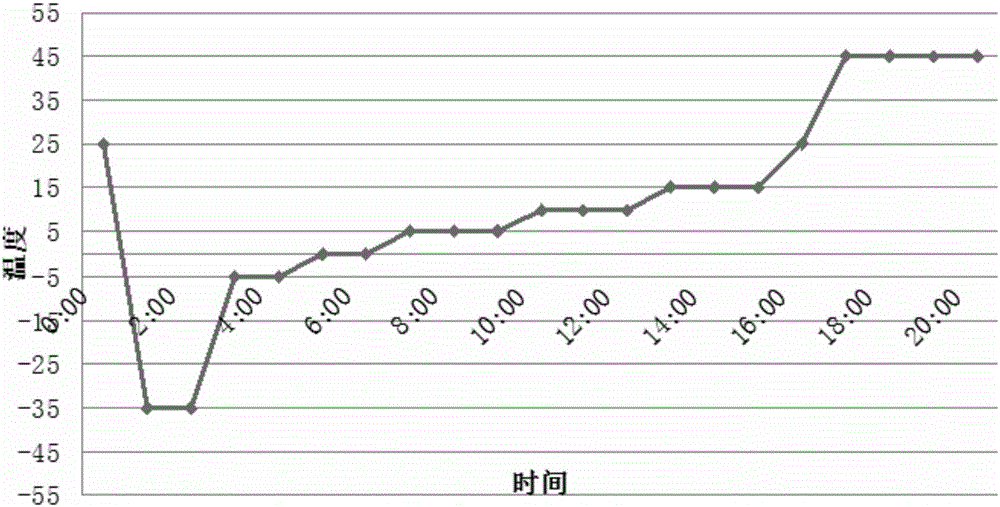
[0042](1) Pre-freezing: transfer the lornoxicam product to the freezer of the lyophilizer, and the lornoxicam product conducts the cold through the plate in the lyophilizer, and adjusts the temperature of the inner plate of the lyophilizer. The temperature of the lornoxicam product on the plate layer drops to -35°C, and it is incubated for 0.75 hours; internal pressure;
[0043] (2) One-time sublimation drying, adopting six-stage stepped heating: a. Directly heat the refrigerant in the slab to -5°C, with a heating rate of 1.5°C / min, and keep warm for 1 hour; when the freezer cavity When the vacuum degree in the body reaches 15 Pa, the pulsating air infiltration is automatically carried out in the cavity of the freezer, and the infiltration is stopped when the vacuum degree in the cavity of the freezer reaches...
Embodiment 2
[0052] Embodiment 2: A kind of lyophilization process of lornoxicam for injection
[0053] Described freeze-drying process comprises the following steps successively:
[0054] (1) Pre-freezing: transfer the lornoxicam product to the freezer of the lyophilizer, and the lornoxicam product conducts the cold through the plate in the lyophilizer, and adjusts the temperature of the inner plate of the lyophilizer. The temperature of the lornoxicam product on the plate layer drops to -32 ℃, and it is incubated for 0.5 hours; internal pressure;
[0055] (2) One-time sublimation drying, adopting six-stage stepped heating: a. Directly heat the refrigerant in the slab to -4°C, the heating rate is 1.5°C / min, and keep warm for 1.5 hours; when the freezer cavity When the vacuum degree in the body reaches 18Pa, the pulsating air infiltration is automatically performed, and the air infiltration is stopped when the vacuum degree in the cavity of the freezer reaches 28Pa;
[0056] b. Heat the...
Embodiment 3
[0064] Embodiment 3: A kind of lyophilization process of lornoxicam for injection
[0065] Described freeze-drying process comprises the following steps successively:
[0066] (1) Pre-freezing: transfer the lornoxicam product to the freezer of the lyophilizer, and the lornoxicam product conducts the cold through the plate in the lyophilizer, and adjusts the temperature of the inner plate of the lyophilizer. The temperature of the lornoxicam product on the plate layer drops to -40°C, and it is incubated for 1 hour; internal pressure;
[0067] (2) Primary sublimation drying, adopting six-stage stepped heating: a. Directly heat the refrigerant in the slab to -6°C, with a heating rate of 1.0°C / min, and keep warm for 1.5 hours; when the freezer cavity When the vacuum degree in the body reaches 12Pa, the pulsating gas infiltration is automatically performed, and the gas infiltration is stopped when the vacuum degree in the cavity of the freezer reaches 22Pa;
[0068] b. Heat the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com