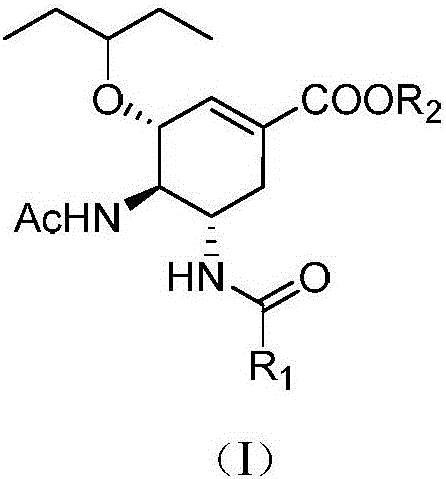
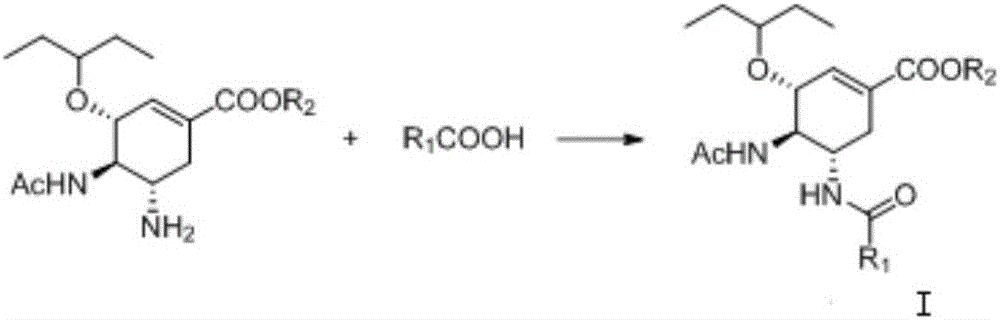
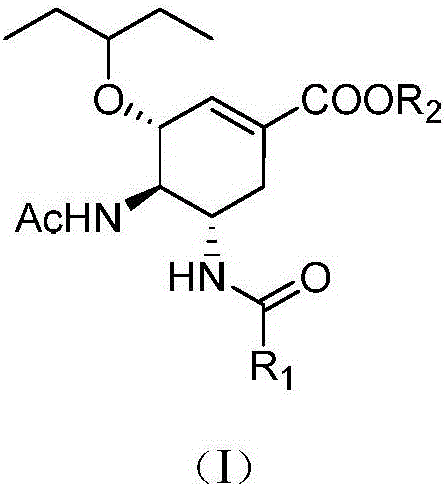
Acyl neuraminidase inhibitor and medical application thereof
An acetamido, pharmaceutical technology, applied in the preparation of carboxylic acid amides, antiviral agents, preparation of organic compounds, etc., can solve the problems of central nervous system toxic and side reactions, easy to produce drug resistance and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (3R,4R,5S)-4-Acetamido-5-acetylamino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethyl ester (Ⅱ-1)
[0033] Add 130mg (0.417mmol) oseltamivir, 238mg HATU (0.625mmol), 182μL DIPEA (1.04mmol), 30mg glacial acetic acid (0.50mmol) to the 50mL round bottom flask, stir the reaction at room temperature, after the reaction, organic phase It was washed with 1N HCl solution, saturated sodium carbonate solution and saturated sodium chloride solution, dried over anhydrous sodium sulfate, concentrated, and purified by column chromatography to obtain 110 mg of solid with a yield of 74.5%.
Embodiment 2
[0035] (3R,4R,5S)-4-Acetamido-5-acetylamino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid (Ⅰ-1)
[0036] Get the previous step product (3R, 4R, 5S)-4-acetylamino-5-acetylamino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethyl ester (Ⅱ-1 )
[0037] 110mg (0.31mmol), add methanol 10mL and 0.78mL 1N sodium hydroxide aqueous solution, add 1.22mL deionized water, make V (methanol): V (water) = 5: 1, stir the reaction at room temperature, after the end of the reaction, reduce Methanol was removed by pressure steaming, and the pH was adjusted to 1-2. The precipitate was precipitated, filtered, and dried to obtain 80 mg of white solid with a yield of 82.6%. 1 H NMR (400MHz, DMSO) δ12.56(s, 1H), 7.77(d, J=9.2Hz, 1H), 7.66(d, J=8.9Hz, 1H), 6.60(s, 0H), 4.07(d ,J=8.3Hz,1H),3.88(m,1H),3.78–3.66(m,1H),3.42–3.35(m,1H),2.49–2.41(m,1H),2.19-2.11(m,0H ),1.77(s,3H),1.76(s,3H),1.47-1.33(m,4H),0.83(t,J=7.4Hz,3H),0.76(t,J=7.4Hz,3H); MS (ESI):349.2[M+Na] + ,325.0[M-H] - .
Embodiment 3
[0039] (3R,4R,5S)-4-Acetamido-5-trifluoroacetylamino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethyl ester (Ⅱ-2)
[0040] The operation is the same as in Example 1, with oseltamivir and trifluoroacetic acid as the reaction raw materials. 43mg of white solid was obtained, the yield was 18%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com