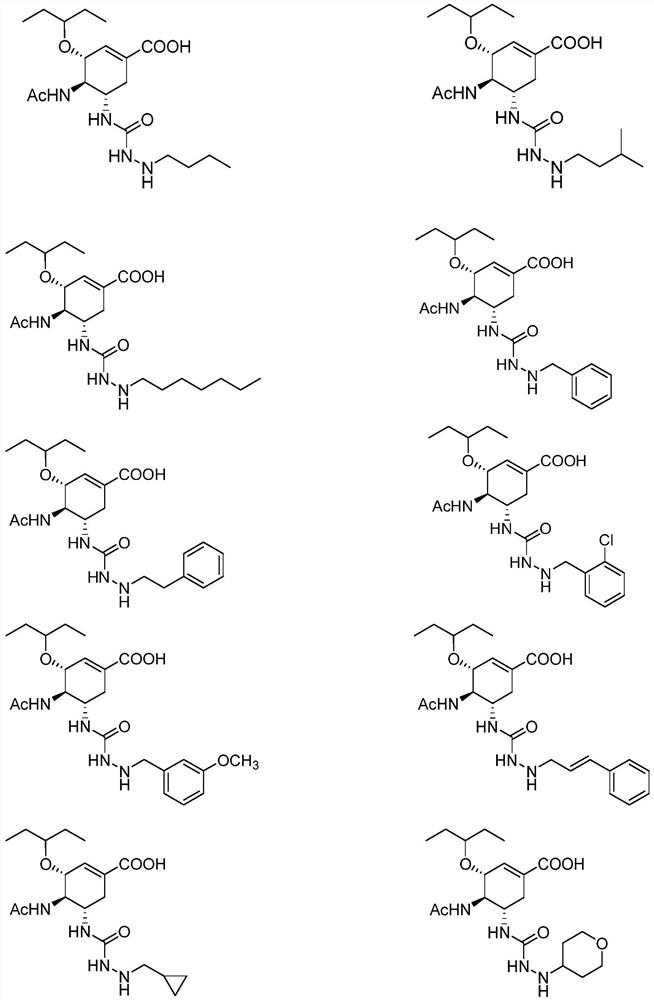
Neuraminidase inhibitor containing hydrazide structure fragments and medical application thereof
An acetamido, pharmaceutical technology, applied in the field of medicinal chemistry, can solve problems such as central nervous system toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
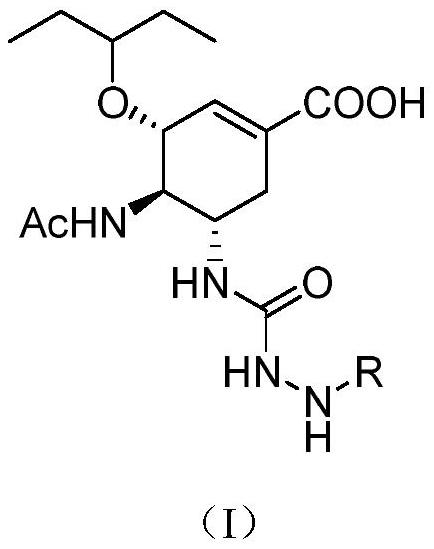
[0038] ((3R, 4R, 5S)-4-acetamido-5-(1-(imidazole)-aminocarboxamido)-3-(1-ethylpropoxy)-1-cyclohexene-1 - Ethyl carboxylate (II)
[0039] Add 500 mg (1.60 mmol) of oseltamivir to a 100 mL round bottom flask, add 30 mL of dichloromethane to dissolve, add 260 mg (1.60 mmol) of carbonyldiimidazole in batches under ice-water bath, and stir. After the reaction, the organic phase was washed with saturated ammonium chloride solution and saturated sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated to obtain 530 mg of crude product, which was further purified and put into the next reaction.
Embodiment 2
[0041] (3R,4R,5S)-4-Acetamido-5-(hydrazinecarboxamido)-3-(pentane-3-oxyl)cyclohex-1-ene-1-carboxylic acid ethyl ester (III)
[0042] Add compound 500mg (1.23mmol) (3R, 4R, 5S)-4-acetamido-5-(1-(imidazole)-aminoformamido)-3-(1-ethyl) in a 100mL round bottom flask Propoxy)-1-cyclohexene-1-carboxylate ethyl ester (II), add 1.92g of anhydrous sodium sulfate (13.52mmol), add 30mL of anhydrous chloroform, 132μL of hydrazine hydrate (2.71mmol), 255μL of three Ethylamine (1.85mmol) was reacted at room temperature for 0.5h, then heated to 35°C for 12h. After the reaction was completed, anhydrous sodium sulfate was removed by filtration, and chloroform was evaporated from the filtrate under reduced pressure to obtain a crude product, which was then slurried by adding 20 mL of acetonitrile to obtain 340 mg of a white solid with a yield of 80.0%.
Embodiment 3
[0044] (3R, 4R, 5S)-4-Acetamido-5-(2-methylenehydrazine-1-carboxamido)-3-(pentane-3-oxyl)cyclohex-1-ene-1- Ethyl carboxylate (IV-1)
[0045] Add compound 200mg (0.58mmol) (3R, 4R, 5S)-4-acetylamino-5-(hydrazine carboxamido)-3-(pentane-3-oxyl group) cyclohexyl-1 in 50mL round bottom flask -Ethyl-ene-1-carboxylate (III), sequentially add 10 mL of methanol, add 25.86 μL of formaldehyde (0.70 mmol), and stir at room temperature for 8 h. After the reaction, the methanol was distilled off under reduced pressure and purified by column chromatography to obtain 201 mg of a white solid with a yield of 90.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com