A kind of preparation method of pentafluorophenol
A technology of pentafluorophenol and toluene, applied in the field of preparation of pentafluorophenol, can solve the problems of high manufacturing cost, waste of bromine resources, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
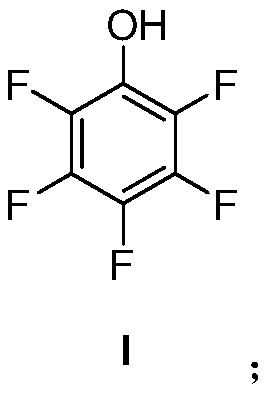
[0053] The first aspect of the present invention provides a kind of preparation method of pentafluorophenol, the structure of described pentafluorophenol is as shown in formula I:
[0054]
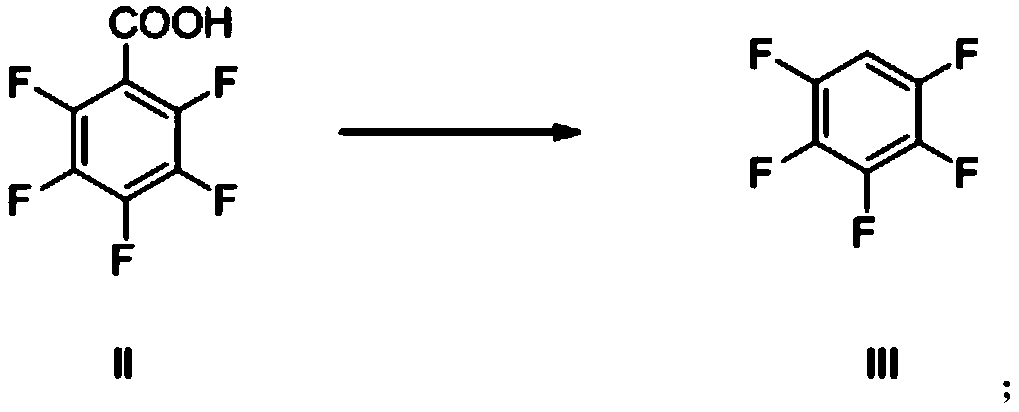
[0055] The preparation method of pentafluorophenol provided by the present invention can comprise decarboxylation reaction, and described decarboxylation reaction is specifically: the compound of formula II is under the condition that alkali exists, generates the compound of formula III, and reaction equation is as follows:
[0056]
[0057] In the decarboxylation reaction, the reaction can usually be carried out under the condition of gas protection, and the gas protection can use one or more combinations of nitrogen, inert gas, etc., and the inert gas can be, for example, helium, neon , argon, krypton, xenon, etc. one or more combinations.
[0058] In the decarboxylation reaction, the reaction can be carried out in the presence of a reaction solvent, which can be an organic solvent...
Embodiment 1
[0083] 1L three-necked flask, add 424 grams of pentafluorobenzoic acid, 1060 grams of toluene, and 202 grams of triethylamine. Heating to 100°C-110°C, reacting for 24 hours, the conversion is complete. Atmospheric pressure recovery triethylamine and toluene. Distilled to obtain 335g of pentafluorobenzene, GC area normalization method purity (A%): 99%, yield: 99%. GCMS: 168.
Embodiment 2-6
[0085] The consumption and kind of organic solvent are as shown in table 1, and other is with embodiment 1, and its result is shown in table 1
[0086] Table 1
[0087]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com