A kind of polypeptide and its application
A technology of peptides and amino acids, applied in the biological field, to achieve the effect of lowering blood sugar, improving the function of pancreatic islets, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 The preparation of epitope polypeptide of the present invention and its vaccine
[0041] The epitope polypeptide of the present invention is obtained by analyzing the epitope sequence of the IL-1β protein. The amino acid sequence of the polypeptide is: SEQ ID NO.1: VQGEESNDKESVDPKNYPKKKMEKRFVFNKIEINNKLEF, and the polypeptide is fully synthesized and purified.
[0042] Vaccine (Al adjuvant) group: After the epitope polypeptide is dissolved in PBS, it is blown and mixed with the aluminum adjuvant for injection at a volume ratio of 3:1. Vaccine (PLA microsphere adjuvant) group: Mix 5 mg / mL PLA with epitope polypeptide, suspend overnight at 4°C. Adjuvant control group: PBS solution and adjuvant were mixed by pipetting at a volume ratio of 3:1. Get the vaccine.
experiment example 2
[0043] Experimental Example 2 Effect of IL-1β Epitope Vaccine on Mouse Antibody Titer
[0044] Mice were injected subcutaneously on days 0, 14, and 28 at biweekly intervals. Each mouse in the vaccine group was injected with a vaccine containing 50 μg of epitope polypeptide. Adjuvant control group and C57 control group were injected with an equal volume of adjuvant solution without epitope polypeptide.
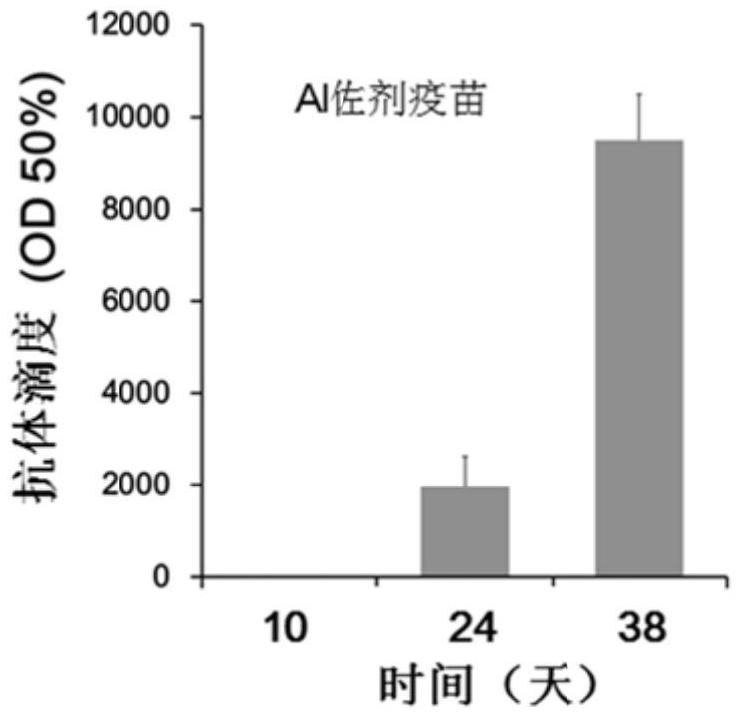
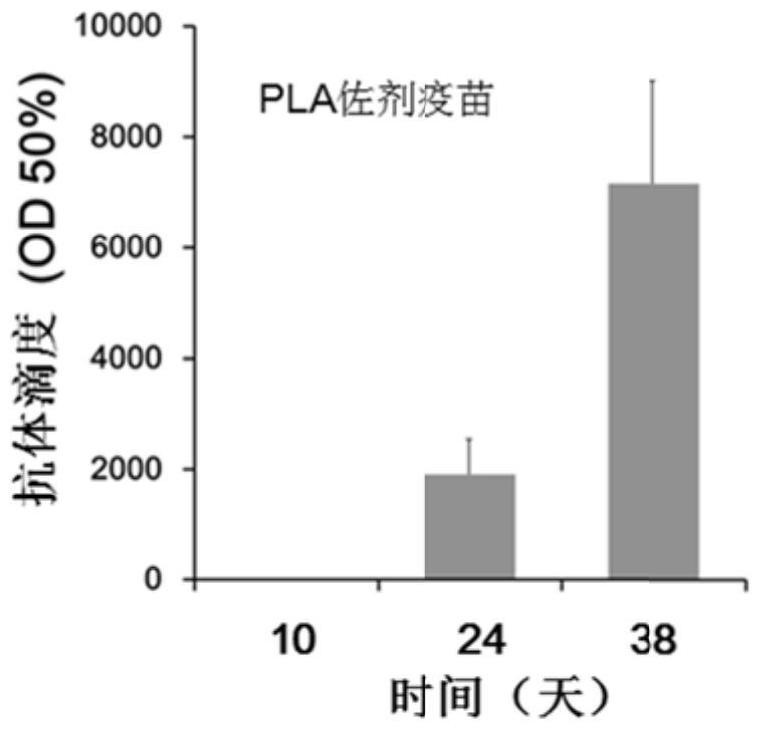
[0045] Determination of antibody titer: Blood was collected from the tail vein on the 10th, 24th and 38th day after the first immunization, and the blood was left to stand at 37°C for 2 hours, centrifuged at 4000r / min for 5min, and the upper serum was taken and packed for use .
[0046] 100 μL of epitope peptides were coated with 0.5 μg / well of 96-well ELISA microplate, BSA was used as negative control, placed at 37°C for 2 hours, blocked with 5% BSA at 37°C for 2 hours, 340 μL / well. The plate was washed 3 times with PBS, 100 μL of mouse serum serially diluted with 5% BSA wa...
experiment example 3
[0048] Experimental example 3 IL-1β epitope vaccine to KK-A y Effects on blood glucose levels in mice
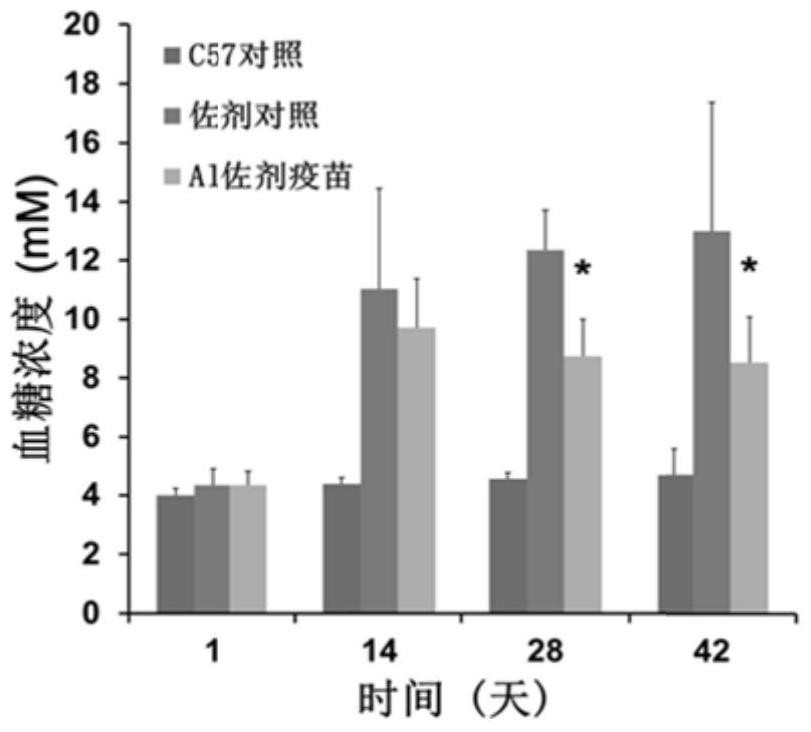
[0049] Blood glucose measurement: The fasting blood glucose of the mice was measured with a Roche blood glucose meter / vigor type on days 0, 14, 28, and 42 after the first immunization at biweekly intervals.
[0050] The blood glucose levels of mice in each group were as follows: Figure 2(A) and 2(B) As shown, the average fasting blood glucose of the C57 control group on days 0, 14, 28, and 42 were 4.0, 4.4, 4.6, and 4.7 respectively; the average fasting blood glucose of the adjuvant control group on days 0, 14, 28, and 42 were 4.3, 11.0, 12.3, 13.0; the average fasting blood glucose of the vaccine group (Al) on days 0, 14, 28, and 42 were 4.3, 9.7, 8.7, 8.5; the vaccine group (PLA) on days 0, 14, 28, The average fasting blood sugar in 42 days was 4.3, 8.1, 8.8, 9.1 respectively; the experimental results showed that KK-A y The blood glucose level of mice increases rapidly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com