Active amino-containing hydantoin derivative copolymeric material and preparation method thereof
A technology of hydantoin derivatives and aminohydantoin, which is applied in the field of polymer synthesis, can solve the problems of inconvenient immobilization of hydantoin derivatives, and achieve excellent broad-spectrum bactericidal performance, mild reaction conditions, and strong bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
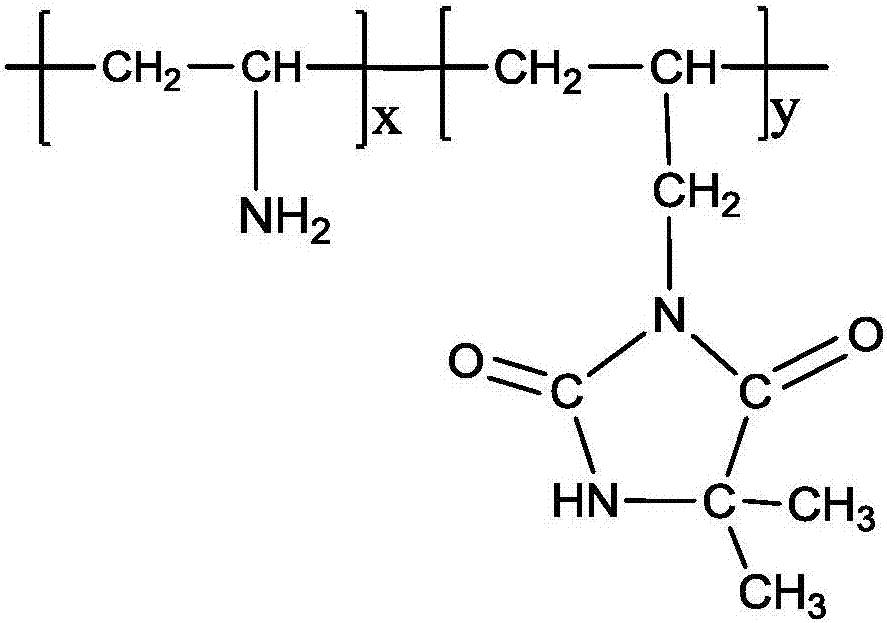
[0019] Weigh 2.5 mol of 3-allyl-5,5-dimethylhydantoin (ADMH), 0.5 mol of N-vinylformamide (NVF) and 0.008 mol of initiator AIBA in proportion, mix them evenly and add to a stirring device Add 4 mol of deionized water to a 2L reactor, start stirring at 60r / min, and pass in nitrogen as a protective gas; adjust the temperature of the reactor to 40°C by controlling the temperature of the circulating water bath, and react for 6h. Add 0.08 mol of concentrated hydrochloric acid (37.5%) and 5 mol of deionized water into the reactor for acidic hydrolysis for 2 hours. The reaction liquid was taken out and poured into 5L of absolute ethanol to precipitate a precipitate. The precipitate was exchanged with 717 strong basic resin to obtain the final copolymerized material. The structural formula of vinylamine-co-3-allyl-5,5-dimethylhydantoin (p(ADMH-co-VAm)) is as follows:
[0020]
[0021] Wherein: X=60000; Y=40000.
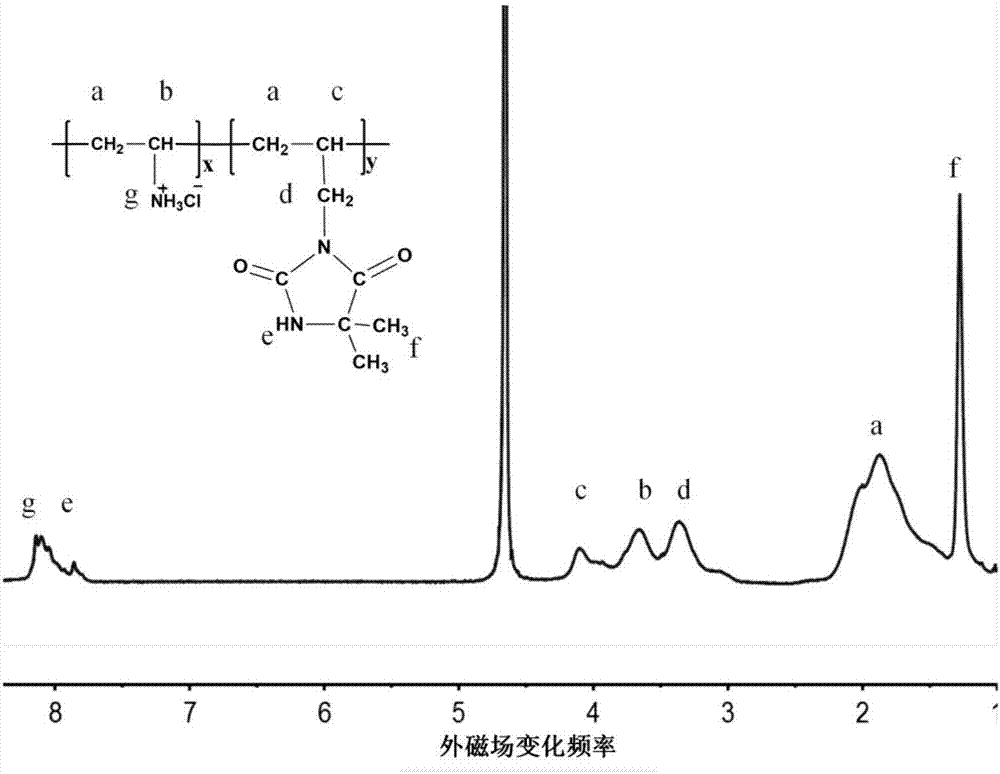
[0022] Such as figure 1 Shown: NMR 1 H NMR (D 2 O): 1.30–2.23pp...
Embodiment 2
[0029] Weigh 0.3 mol of 3-styryl-5,5-dimethylhydantoin (VBDMH), 5.2 mol of acrylamide (AM) and 0.008 mol of initiator AIBA in proportion, mix them evenly and add them to the reaction kettle equipped with a stirring device , add 35 mol of deionized water into a 2L reactor, start stirring at 105r / min, pass nitrogen gas as a protective gas; adjust the temperature of the reactor to 45°C by controlling the temperature of the circulating water bath, and react for 6h. Add 0.50 mol of concentrated hydrochloric acid (37.5%) and 2 mol of deionized water into the reactor for acidic hydrolysis for 1.5 h. The reaction liquid was taken out and poured into 3L of absolute ethanol to precipitate a precipitate. The precipitate was exchanged with 717 strong basic resin to obtain the final copolymerized material. The structural formula of acrylamine-co-3-styryl-5,5-dimethylhydantoin (p(VBDMH-co-Am)) is as follows:
[0030]
[0031] Wherein: X=90000; Y=10000.
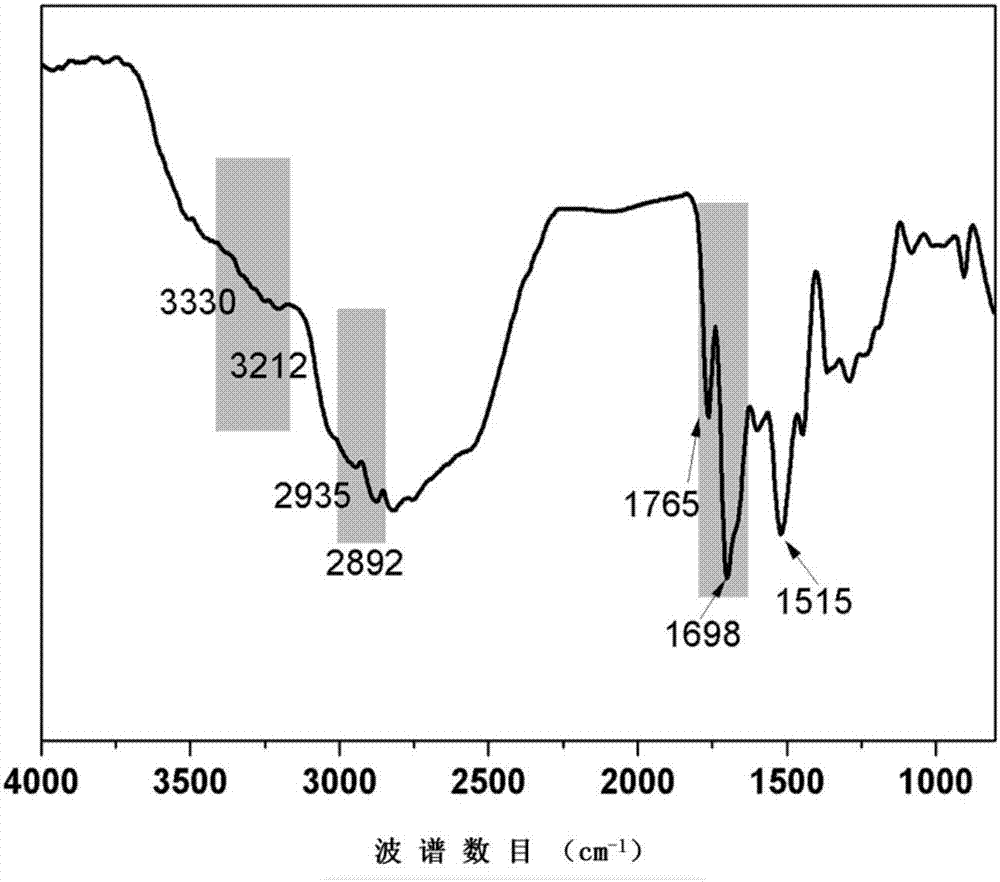
[0032] NMR and infrared charac...
Embodiment 3
[0034] Weigh 2.5 mol of 3-allyl-5,5-dimethylhydantoin (ADMH), 4.6 mol of methacrylamide (MAM) and 0.025 mol of initiator AIBA in proportion, mix them evenly and add to the reaction mixture equipped with a stirring device. In the kettle, add 25mol deionized water into the 2L reactor, start stirring at 60r / min, and pass in nitrogen as a protective gas; adjust the temperature of the reactor to 50°C by controlling the temperature of the circulating water bath, and react for 6h. Add 0.50 mol of concentrated hydrochloric acid (37.5%) and 15 mol of deionized water into the reactor for acidic hydrolysis for 2.5 hours. The reaction liquid was taken out and poured into 5L of absolute ethanol to precipitate a precipitate. The precipitate was exchanged with 717 strong basic resin to obtain the final copolymerized material. The structural formula of methacrylamine-copolymerized-3-allyl-5,5-dimethylhydantoin (p(ADMH-co-MAm)) is as follows:
[0035]
[0036] Wherein: X=58000; Y=45000. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com