Method for improving stability of indapamide tablets in acidic solution
A technology for indapamide tablets and acidic solutions, which is applied in the field of improving the stability of indapamide tablets in acidic solutions, can solve the problems of reduced cumulative dissolution rate and influence on experimental data collection, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] (1) Preparation of acidic solution: Weigh a certain amount of salt and acid, add water and dilute to 1000ml, determine its pH, mix well, and get ready.
[0034] (2) Add appropriate amount of additives, such as vitamin C, to the medium.
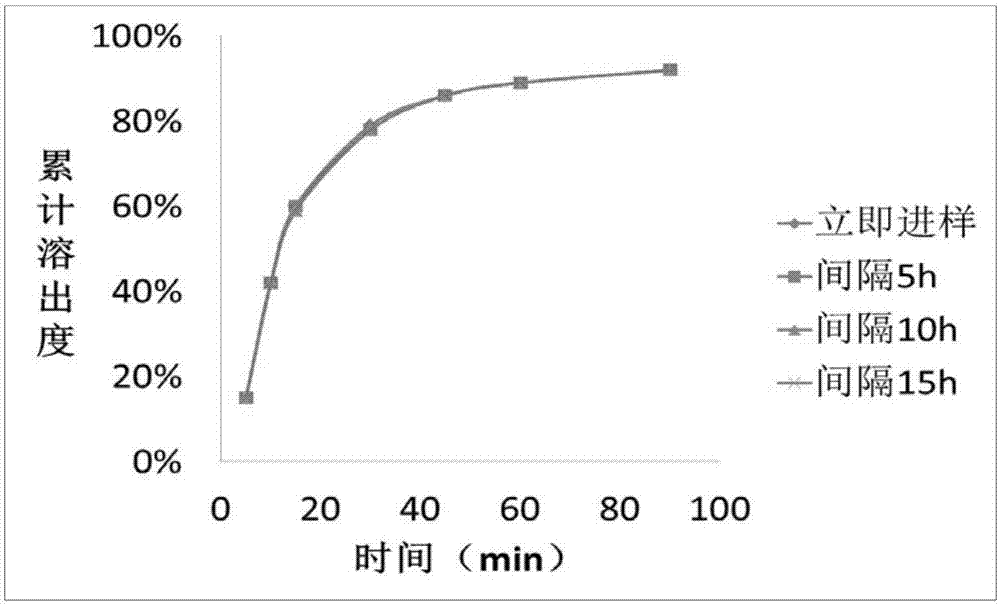
[0035] (3) Get indapamide tablet (2.5mg), add in the above-mentioned prepared medium, the rotating speed is 50 revolutions per minute, after 5min, 10min, 15min, 30min, 45min, 60min, 90min of stripping, take the appropriate amount of solution respectively, Discard 5ml of the initial filtrate, and take the subsequent filtrate as the test solution. Take an appropriate amount of the test solution immediately, respectively, at an interval of 5h, at an interval of 10h, and after an interval of 15h, inject it into a high-performance liquid chromatograph, and record the spectrum.
[0036] (4) Measuring conditions: the stationary phase of high performance liquid chromatography is octadecylsilane bonded silica gel, the mobile phase is 0.1% phosp...
Embodiment 1
[0038] Investigation of the effect of vitamin C concentration on the dissolution of indapamide in an acidic solution of hydrochloric acid at pH 1.2
[0039] The hydrochloric acid solutions of pH 1.2 (wherein the mass content of sodium chloride is 0.2%) were prepared with vitamin C mass concentrations of 0.01%, 0.02%, 0.03%, 0.05%, and 0.1%, respectively. The prepared samples are respectively 1-1#, 1-2#, 1-3#, 1-4#, 1-5#. Other steps are the same.
[0040] The assay results of dissolved indapamide are shown in Table 1.
Embodiment 2
[0042] Investigate the effect of pH value of acidic solution on the dissolution of indapamide in acidic solution of hydrochloric acid
[0043] The preparation pH is respectively the hydrochloric acid solution of 1.0, 1.2, 1.5, 2.2, wherein containing the sodium chloride that mass content is 0.2%, the sample that prepares is respectively 2-1#, 2-2#, 2-3# and 2- 4#. It is 0.02% to prepare vitamin C mass content again, and pH is respectively the hydrochloric acid solution of 1.0,1.2,1.5,2.2, wherein contains the sodium chloride that mass content is 0.2%, the sample that prepares is respectively 2-5#, 2- 6#, 2-7#, 2-8#. Other steps are the same.
[0044] The assay results of dissolved indapamide are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com