Novel use of pyrvinium pamoate in preparation of anti-mycobacterium tuberculosis drugs
A technology of mycobacterium tuberculosis and protozolin, which is applied in the direction of antibacterial drugs, pharmaceutical formulas, and medical preparations containing active ingredients, etc., can solve the problems of no reports on the anti-mycobacterium tuberculosis effect of protozoline, and avoid The effect of long approval time and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
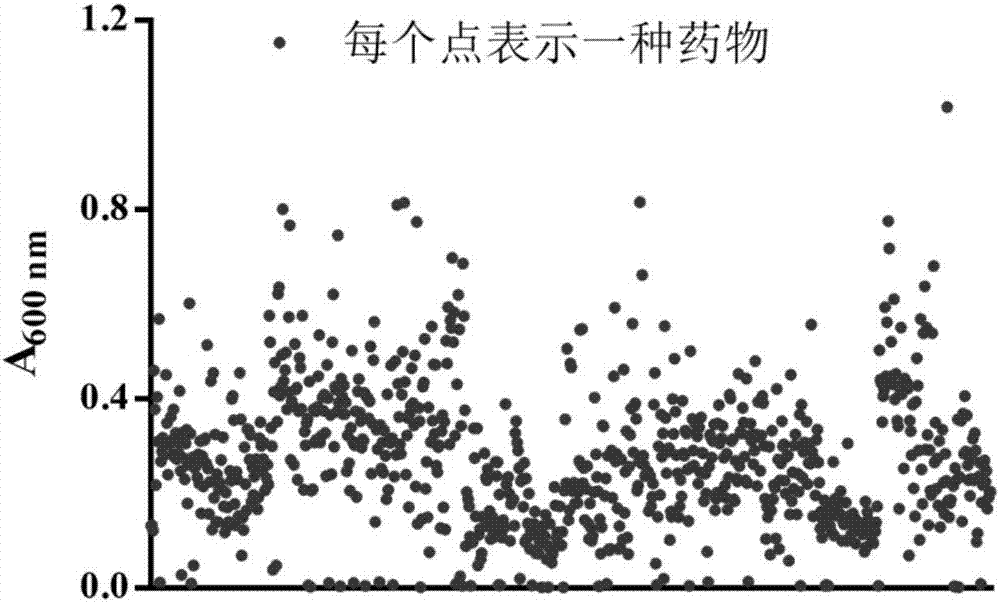
[0031] [Example 1] 60 kinds of Mycobacterium smegmatis bacteriostatic agents were identified in 1280 Chinese drug libraries
[0032] In order to find new inhibitors of Mycobacterium tuberculosis, we used Mycobacterium smegmatis to screen 1280 kinds of drugs to screen new anti-tuberculosis drugs.
[0033] 1) Streak inoculation of Mycobacterium smegmatis strains on 7H10 solid medium, culture upside down at 37°C for 2 days, pick a single colony of Mycobacterium smegmatis and culture it in 7H9 liquid medium, and use enzyme Standard instrument A for the detection of bacteria 600 value;
[0034] 2) Then the bacterial concentration was diluted to 2.5×10 6 CFU / mL, add 200μl per well into 96-well cell culture plate;
[0035] 3) Utilize the 1280 kinds of drug libraries provided by MicroSource Discovery Systems, Inc. (Gaylordsville, CT, USA), and add the drugs to the 96-well cell culture plate through the Echo acoustic wave micropipette system (Labcyte, USA). The concentration duri...
Embodiment 2
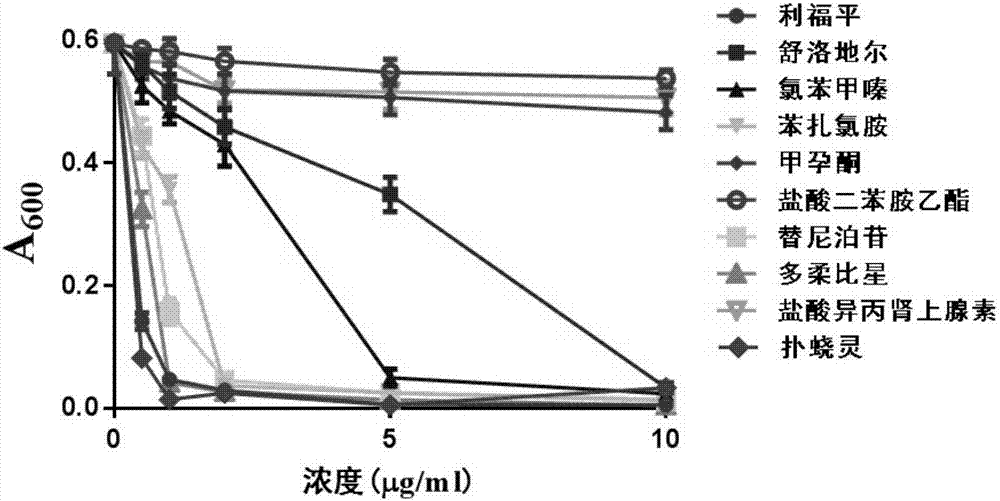
[0040] [Example 2] 6 kinds of medicines have dose-dependent effect on the growth inhibition of Mycobacterium smegmatis
[0041] For the 9 drugs screened in Example 1, continue to use Mycobacterium smegmatis as a model bacterium, and add the above 9 drugs at different concentrations for detection.
[0042] 1) Streak inoculation of Mycobacterium smegmatis strains on 7H10 solid medium, culture upside down at 37°C for 2 days, pick a single colony of Mycobacterium smegmatis and culture it in 7H9 liquid medium, and use enzyme Standard instrument A for the detection of bacteria 600 value;
[0043] 2) Then the bacterial concentration was diluted to 2.5×10 6 CFU / mL, add 200μl per well into 96-well cell culture plate;
[0044] 3) Nine drugs with different concentrations (0, 0.5, 1, 2, 5, 10 μg / ml) were added to the 96-well cell culture plate, and after continuing to culture for 2 days, the A 600 value. Rifampicin was used as a positive control.
[0045] Such as figure 2As show...
Embodiment 3
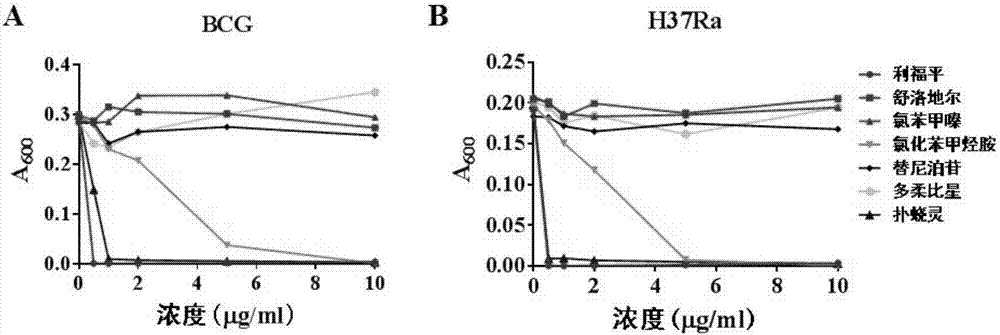
[0046] [Example 3] Two drugs have dose-dependent growth inhibition of BCG and H37Ra
[0047] For the six drugs screened in Example 2, BCG and H37Ra were used as model bacteria, and different concentrations of the above six drugs were added for detection.
[0048] 1) Streak inoculation of BCG and H37Ra strains on 7H10 solid medium, culture upside down at 37°C for 30 days, pick BCG and H37Ra monoclonal colonies and culture them in 7H9 liquid medium, and use a microplate reader after 30 days A for the detection of bacteria 600 value;
[0049] 2) Then the bacterial concentration was diluted to 2.5×10 6 CFU / mL, add 5ml per tube to a 15ml centrifuge tube;
[0050] 3) Add the above 6 drugs at different concentrations (0, 0.5, 1, 2, 5, 10 μg / ml) respectively, and after 30 days, detect the A of the bacterial solution stimulated by different concentrations of drugs. 600 value. Rifampicin was used as a positive control.
[0051] The result is as image 3 As shown, only two drugs ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com