Human DC-CIK immunocompetent cell and preparing method thereof
A technology of DC-CIK and immune activity, applied in the field of human DC-CIK immune activity cells and its preparation, can solve the problem of unsatisfactory curative effect, achieve the effect of improving the curative effect in vivo and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
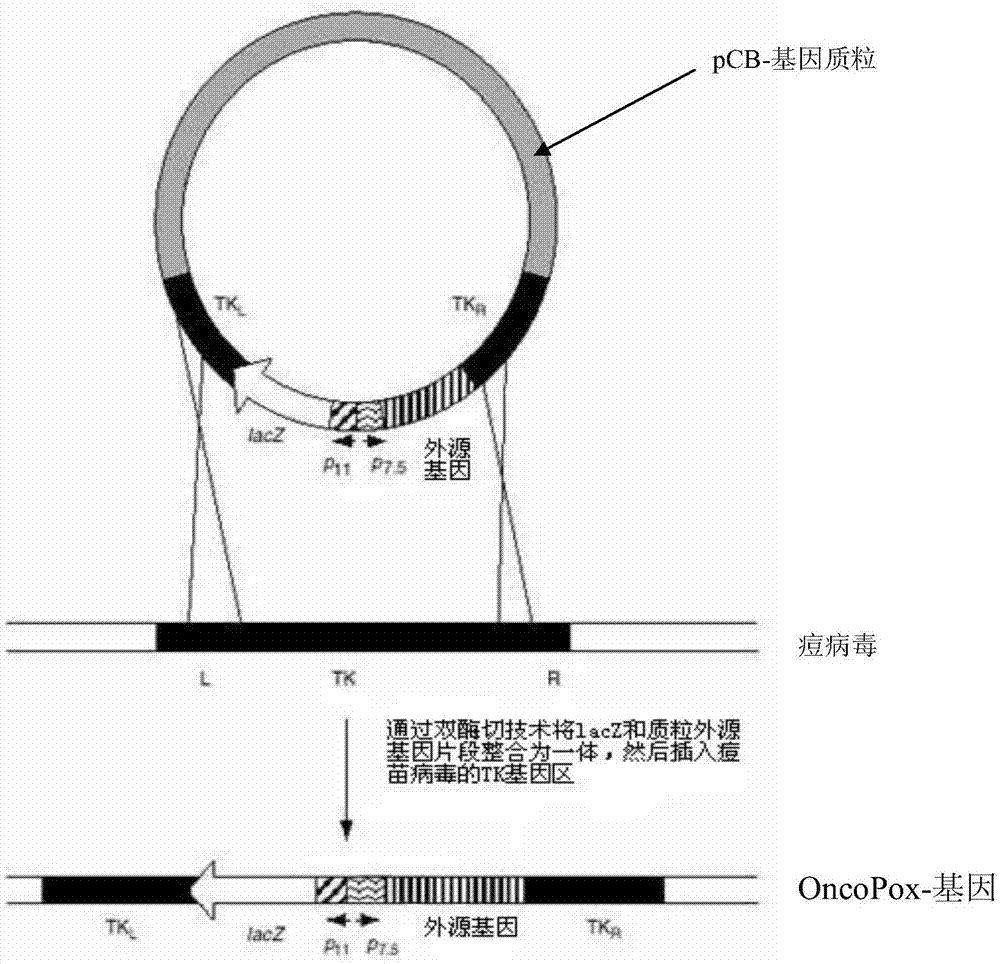
[0058] Example 1 , Construction of vaccinia oncolytic gene virus Oncopox-IL-24
[0059] 1.1. Construction of pCB-IL-24 plasmid
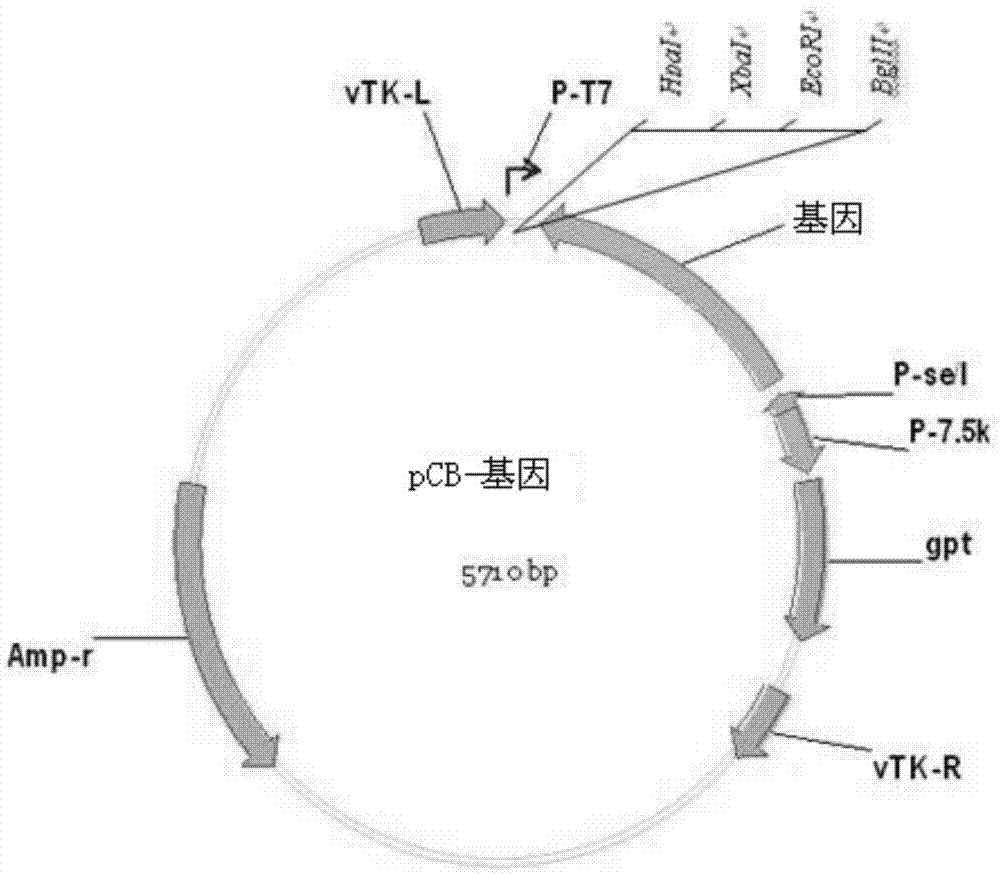
[0060] Synthesize the gene sequence of IL-24 protein, and add BglII and XbaI enzyme cutting sites at both ends of the IL-24 gene sequence, and use restriction endonucleases BglII and XbaI to double-digest the pCB plasmid (Gu Maozhi, Jiang Fumei, Cai Mingjie , Wu Xiangfu, the construction of recombinant plasmid pCB [J], "Progress in Biochemistry and Biophysics", 1980, 7(4):46-48) and the plasmid containing IL-24 gene, using the gel recovery method to recover the large fragment of pCB and The IL-24 gene fragment was purified, and then the two purified gene fragments were mixed together in proportion, then T4 ligase was added, and ligated overnight at 4°C to construct the pCB-IL-24 plasmid. Then by transforming DH5α competent cells, cultured overnight at 37°C. After picking a single clone, expand the culture and extract the corresponding plasmid. S...
Embodiment 2
[0065] Embodiment 2, Identification of vaccinia oncolytic gene virus Oncopox-IL-24
[0066] Use PCR method to detect whether the homologous recombination vaccinia virus contains the target gene, and also to identify whether the homologous recombination vaccinia virus is contaminated by wild virus, design two primers, one primer takes the upstream and downstream of the IL-24 gene Gene sequence, a primer to get the upstream and midstream gene sequence of TK enzyme gene.
[0067] 1) The primers for identifying the target gene are:
[0068] Upstream primer: 5'-CGCGCGTAATACGACTCACT-3',
[0069] Downstream primer: 5'-GAAGGCATCAGTCGGCTTGCG-3',
[0070] 2) The primers for identifying the wild-type virus are:
[0071] Upstream primer: 5'-TGTGAAGACGATAAATTAATGATC-3',
[0072] Downstream primer: 5'-GTTTGCCATACGCTCACAG-3'.
[0073] 3) The PCR reaction system is:
[0074]
[0075] 4) PCR reaction conditions are:
[0076]
[0077] If the PCR product of the viral plaque contai...
Embodiment 3
[0078] Embodiment 3, Amplification of vaccinia oncolytic gene virus Oncopox-IL-24
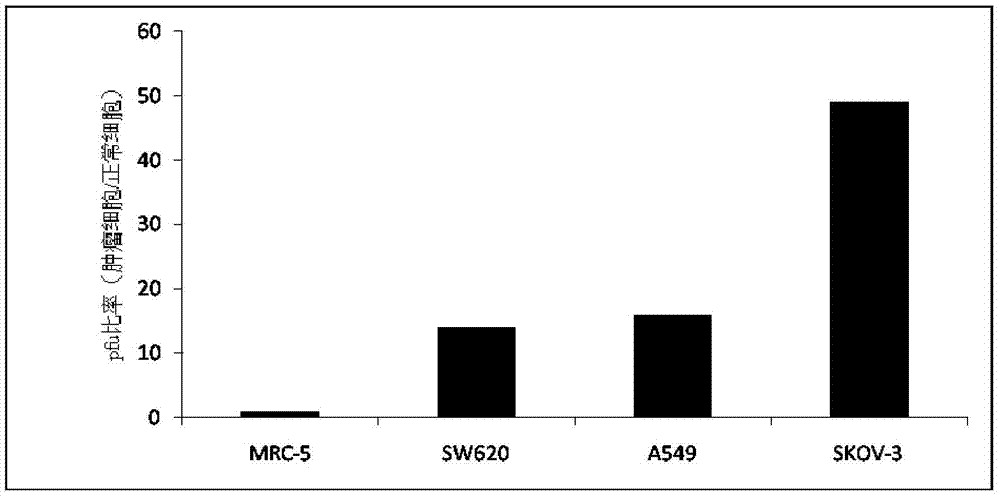
[0079] When the 293 cells grow to about 80% of the culture dish, add a certain amount of vaccinia virus, and then continue to put it in 37 ℃, 5% CO 2 cultured in an incubator. 2 to 3 days after the 293 cells were infected with the virus, the 293 cells were collected and repeatedly frozen and thawed between -80°C and 37°C to lyse the cells and release the virus. Finally, centrifuge using density gradient purification method, and collect the supernatant. For details, refer to the cesium chloride gradient centrifugation purification instructions for viruses (Microbix Biosystem Inc).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com