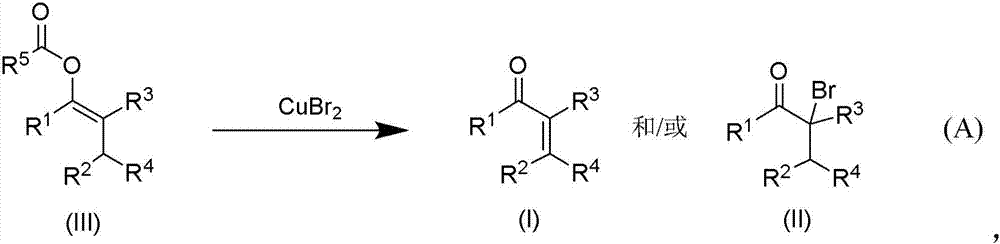
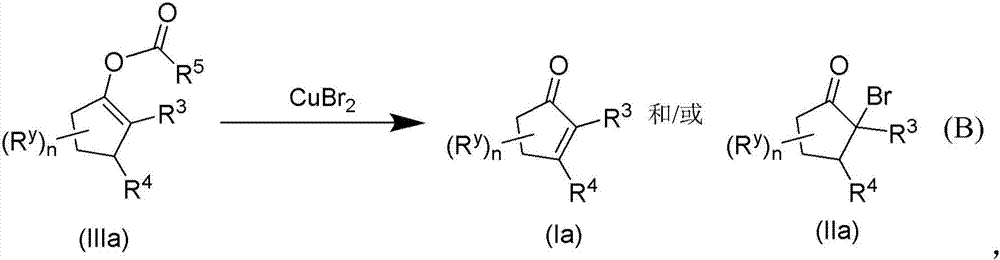
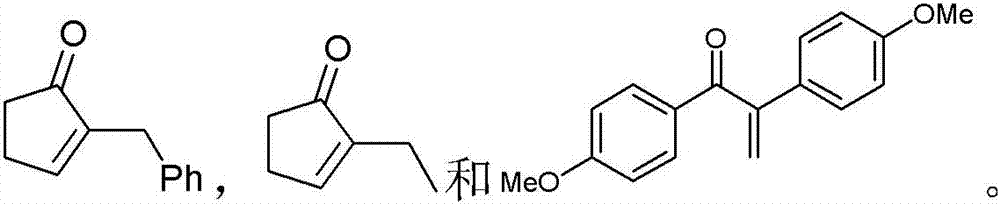
Methods of preparing alpha,beta-unsaturated or alpha-halo ketones and aldehydes
A technology of unsaturated and brominated ketones, which is applied in the field of compounds for producing dehydrogenation and perfume technology, can solve the problems such as unreported enol acetate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0180] Example 1. Methyl 2-(3-acetoxy-2-pentylcyclopent-2-en-1-yl)acetate (1a)
[0181]
[0182] Light brown liquid (5 mmol scale, 1.31 g, 98%). 1 H NMR (400MHz, CDCl 3)δ3.70(s, 3H), 3.07(m, 1H), 2.56(dd, J=4.4, 14.8Hz, 1H), 2.48(m, 2H), 2.14(s, 3H), 2.07-2.24(m , 3H), 1.80(m, 1H), 1.63(m, 1H), 1.42(m, 1H), 1.27(m.5H), 0.90(t, J=7.9Hz, 3H)ppm; 13 C NMR (100MHz, CDCl 3 )δ C 173.3, 168.6, 145.2, 128.3, 51.5, 39.5, 38.6, 31.7, 29.6, 27.1, 26.7, 24.4, 22.4, 20.8, 14.0ppm; FT-IR v max 1008(m), 1204(s), 1368(m), 1436(w), 1737(s), 2930(w)cm -1 ; GC-MS R t 4.79min, m / z 268[M] + , 226[M-Ac] + .
Embodiment 2
[0183] Example 2. 3-Methyl-2-pentylcyclopent-1-en-1-yl acetate (2a)
[0184]
[0185] The starting material was obtained by the organocuprate conjugate addition of 2-pentylcyclopent-2-enone (Ravid, U. and Ikan, R.J.Org.Chem. 1974, 78, 2637-2639). Pale yellow liquid (2 mmol scale, 375 mg, 86%), (3:1 isomer ratio). 1 H NMR (400MHz, CDCl 3 )δ2.70(m, 1H), 2.45(m, 2H), 2.16(s, 3H), 2.15-1.81(m, 2H), 1.51-1.22(m, 8H), 1.05(d, J=6.9Hz , 3H), 0.90 (t, J=7.0Hz, 3H) ppm; 13 C NMR (100MHz, CDCl 3 )δ C 168.8, 143.8, 130.8, 37.3, 31.7, 29.7, 29.4, 26.7, 24.4, 22.4, 20.8, 19.6, 14.0ppm; FT-IR v max 1202(s), 1180(s), 1369(w), 1756(m), 2859(w), 2929(w), 2956(w)cm -1 ; GC-MS R t 3.85min, m / z210[M] + , 168[M-Ac] + .
Embodiment 3
[0186] Example 3. 2-Pentylcyclopent-1-en 1-yl acetate (3a)
[0187]
[0188] The starting material was obtained by hydrogenation of 2-pentylcyclopent-2-enone (aldol product of cyclopentanone and valeraldehyde). Colorless liquid (2.5 mmol scale, 295 mg, 70%), (8:1 isomer ratio). 1 H NMR (400MHz, CDCl 3 )δ2.48(m, 2H), 2.31(m, 2H), 2.17(s, 3H), 2.03-1.88(m, 4H), 1.42-1.21(m, 6H), 0.90(t, J=7.1Hz , 3H)ppm. 13 C NMR (100MHz, CDCl 3 )δ C 168.9, 143.8, 126.9, 31.6, 31.1, 31.0, 26.8, 26.4, 22.5, 20.8, 19.8, 14.0ppm; FT-IR v max 1210(s), 1739(s), 2859(w), 2930(m), 2956(m)cm -1 ;GC-MSR t 3.76min, m / z 196[M] + , 154[M-Ac] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com