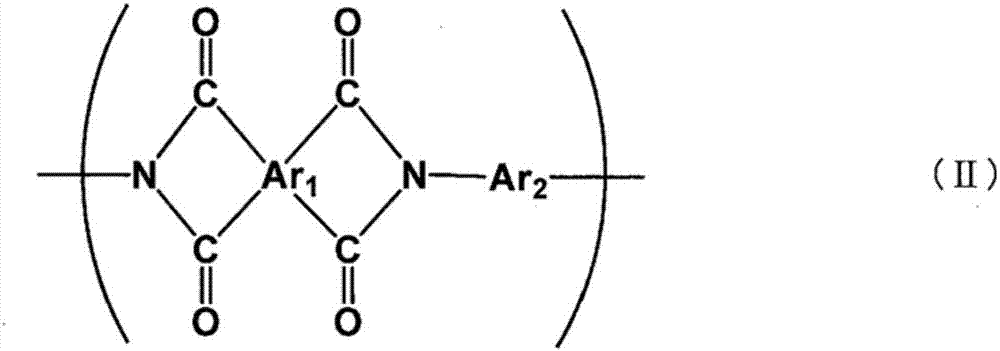
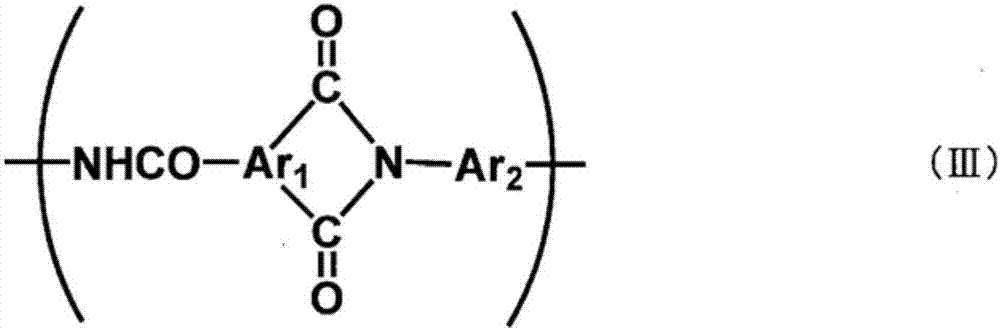
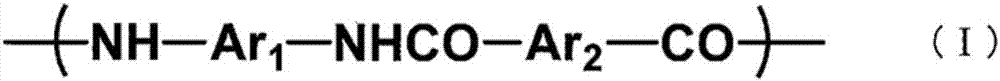Polymer-ion-permeable membrane, composite-ion-permeable membrane, battery electrolyte membrane, and electrode composite body
An ion-permeable membrane and polymer technology, applied in the direction of non-aqueous electrolyte battery electrodes, battery electrodes, non-aqueous electrolyte batteries, etc., can solve low conductivity, elastic modulus, reduced heat resistance, and damaged contact inhibition function and other problems, to achieve the effects of deformation resistance, impact resistance, excellent flexibility, and excellent safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Dissolve 2,2'-bis(trifluoromethyl)-4,4'-diaminobiphenyl (manufactured by Toray Fine Chemical Co., Ltd.) as a diamine in dehydrated N-methyl-2- In pyrrolidone (NMP, manufactured by Mitsubishi Chemical Corporation), it was cooled to 30° C. or lower. 2-Chloroterephthaloyl chloride (manufactured by Nippon Light Metal Co., Ltd.) corresponding to 99 mol% relative to the total amount of diamine was added thereto over 30 minutes while the system was kept under a nitrogen stream at 30° C. After adding the total amount, stirring was performed for about 2 hours to polymerize the aromatic polyamide (A). The obtained polymerization solution was neutralized with 97 mol% of lithium carbonate (manufactured by Honso Chemical Co., Ltd.) and 6 mol% of diethanolamine (manufactured by Tokyo Chemical Industry Co., Ltd.) with respect to the total amount of acid chlorides, and an aromatic polyamide (A) was obtained. The solution. The logarithmic viscosity η of the obtained aromatic polyamide...
Embodiment 2
[0150] The diamine used to obtain the aromatic polyamide (B) is 2,2'-bis(trifluoromethyl)-4,4'-diaminobiphenyl equivalent to 70 mol% relative to the total amount of diamine and equivalent In 30 mol% of 1,3-phenylenediamine (manufactured by Tokyo Chemical Industry Co., Ltd.), the acid chloride was terephthaloyl chloride (manufactured by Tokyo Chemical Industry Co., Ltd.) corresponding to 99 mol% of the total amount of diamine, except Except that, a sample of the polymer ion-permeable membrane was obtained in the same manner as in Example 1. Table 1 and Table 2 show the evaluation results of the obtained samples.
Embodiment 3
[0152] The acid chloride used to obtain the aromatic polyamide (C) was 2-fluoroterephthaloyl chloride (manufactured by Iharanickei Chemical Industry Co., Ltd.) corresponding to 99 mol% relative to the total amount of diamines. Samples of polymer ion-permeable membranes were obtained in the same manner. Table 1 and Table 2 show the evaluation results of the obtained samples.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| gas permeability | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap