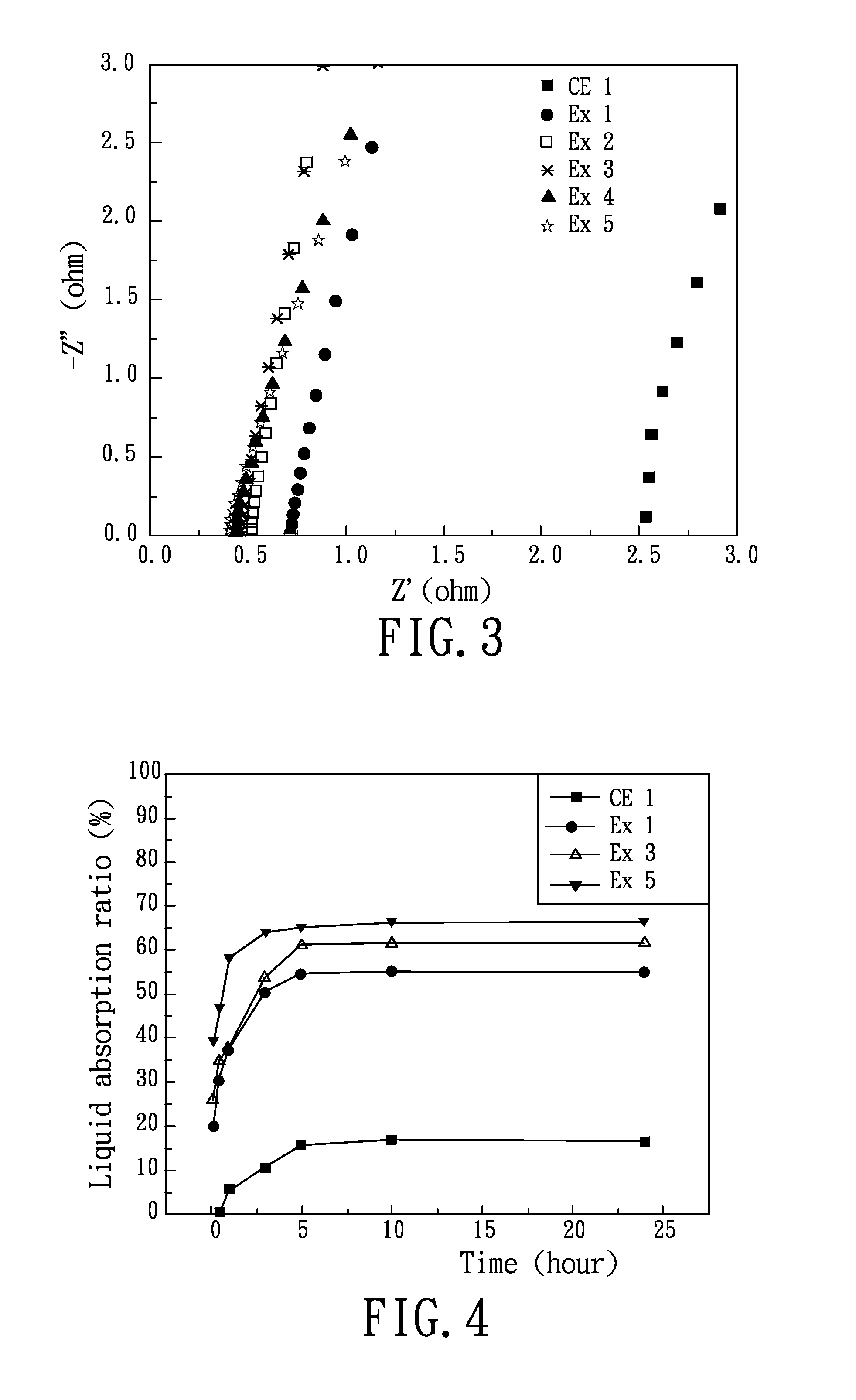
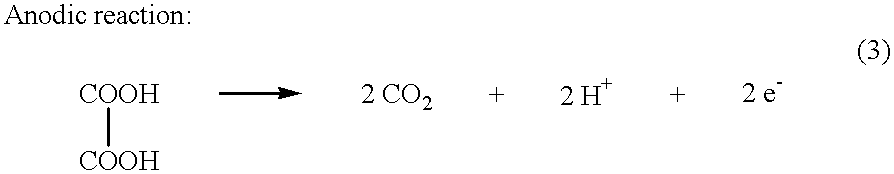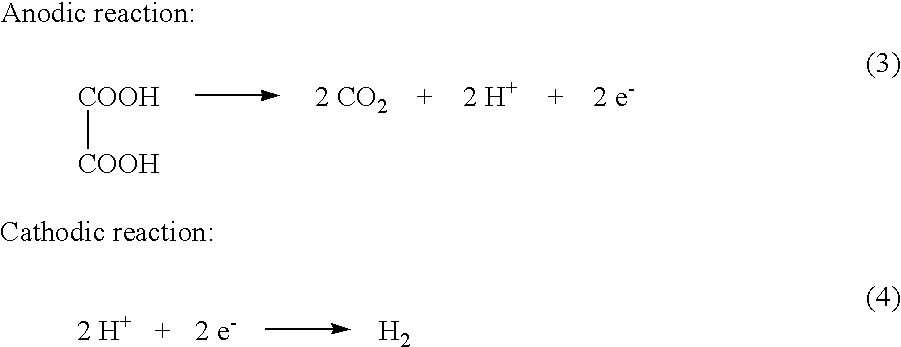Patents
Literature
86 results about "Ion-permeable membranes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
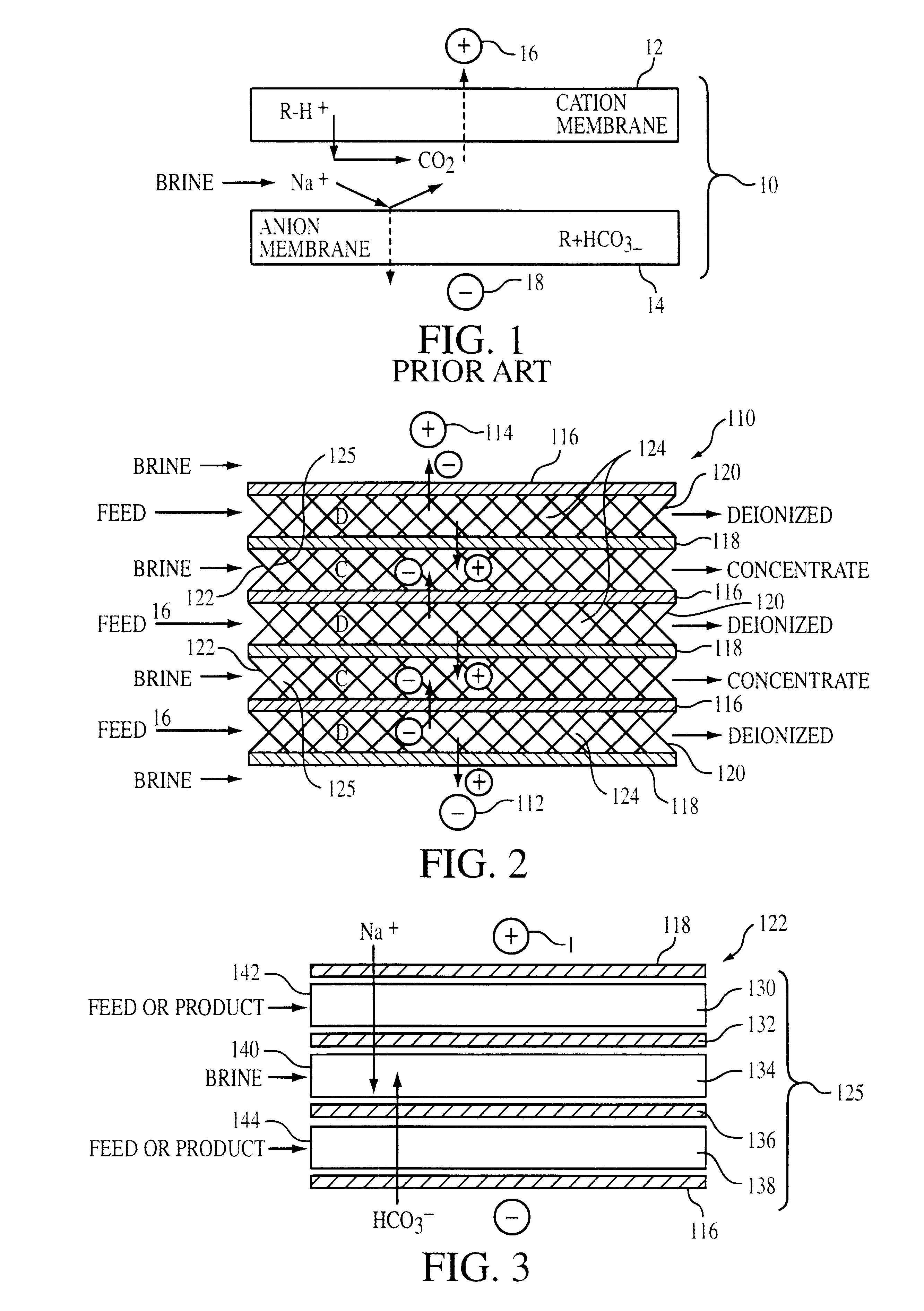
The ion permeable membranes used in electrodialysis are essentially sheets of ion-exchange resins. They usually also contain other polymers to improve mechanical strength and flexibility.
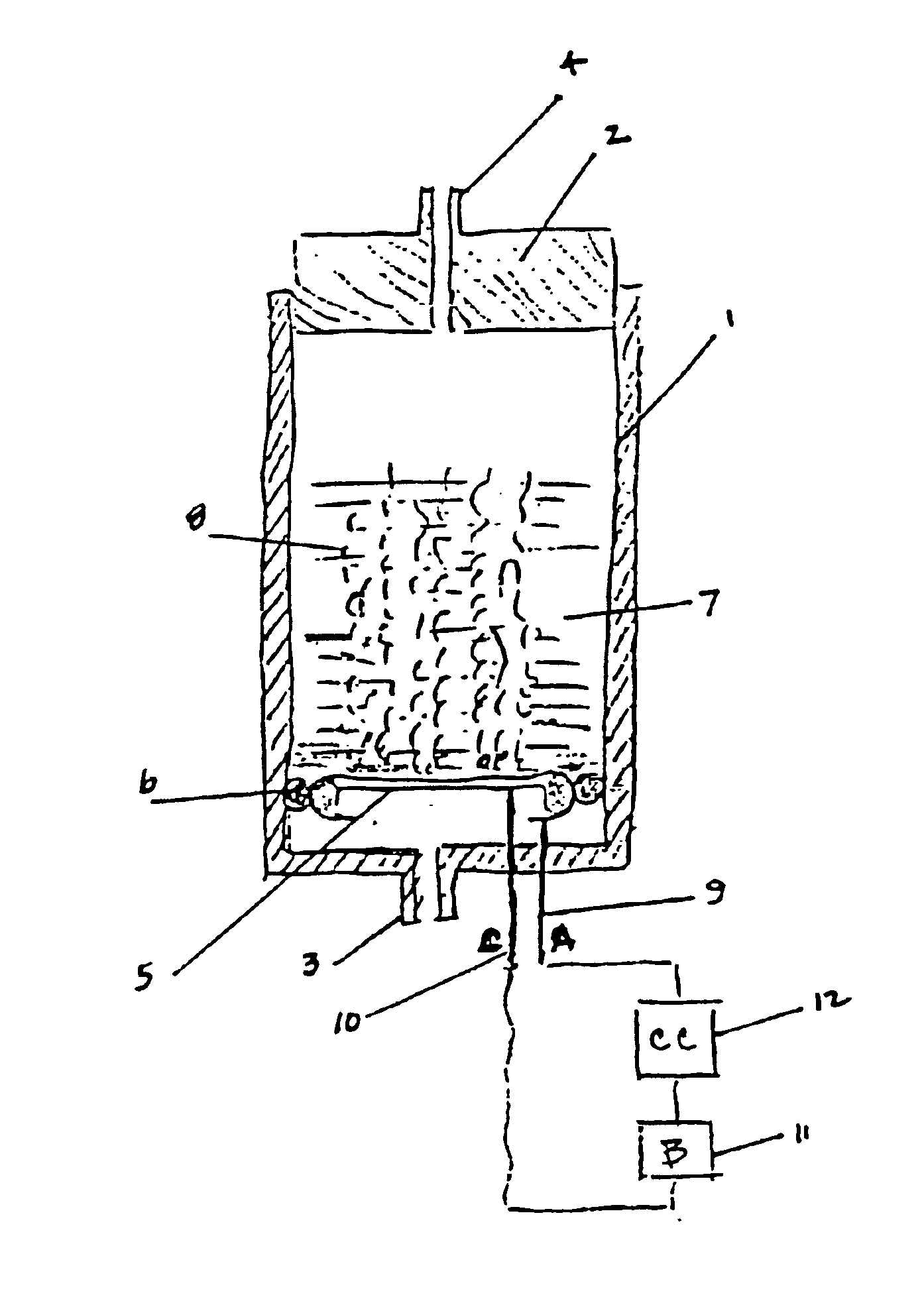
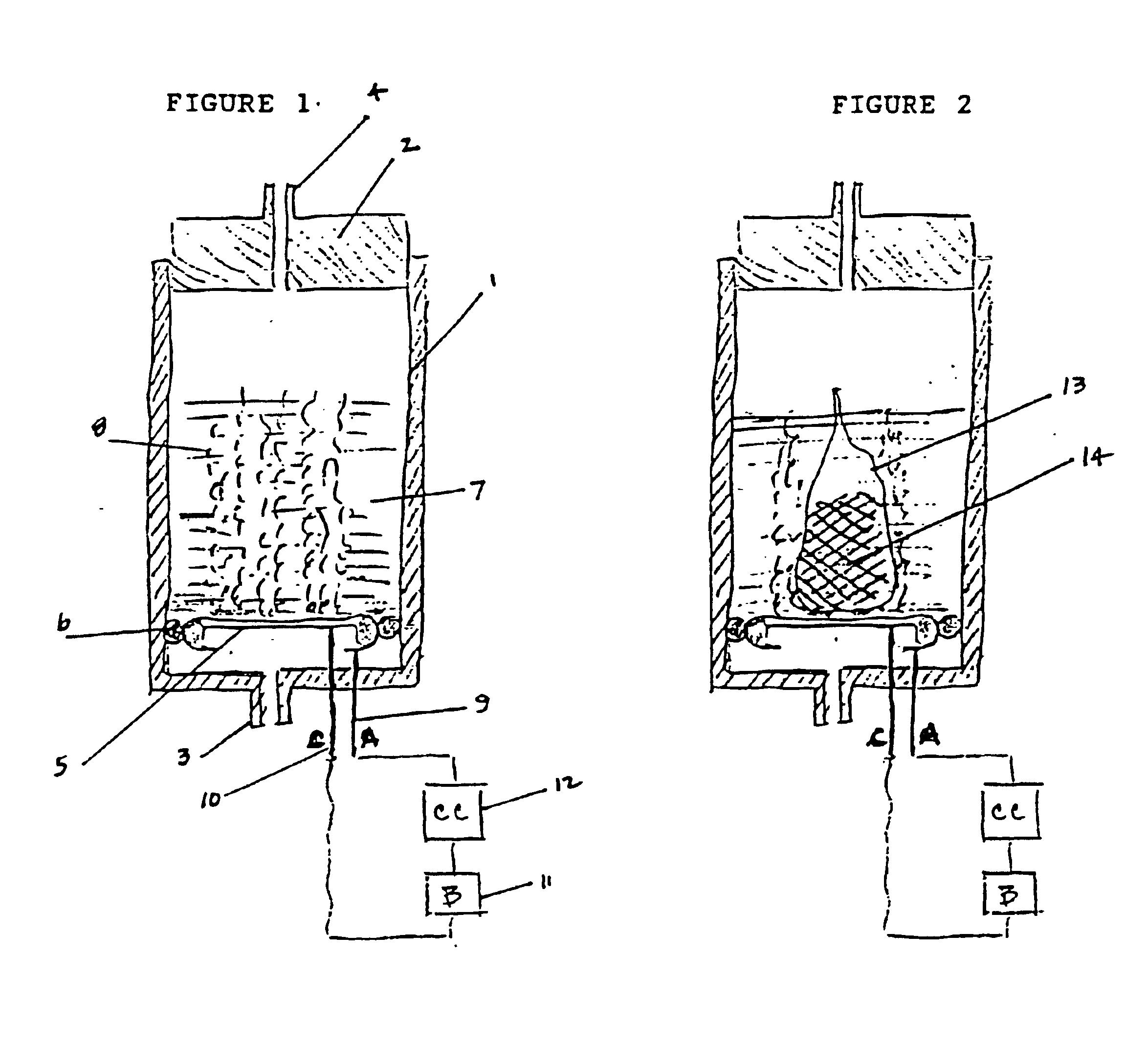
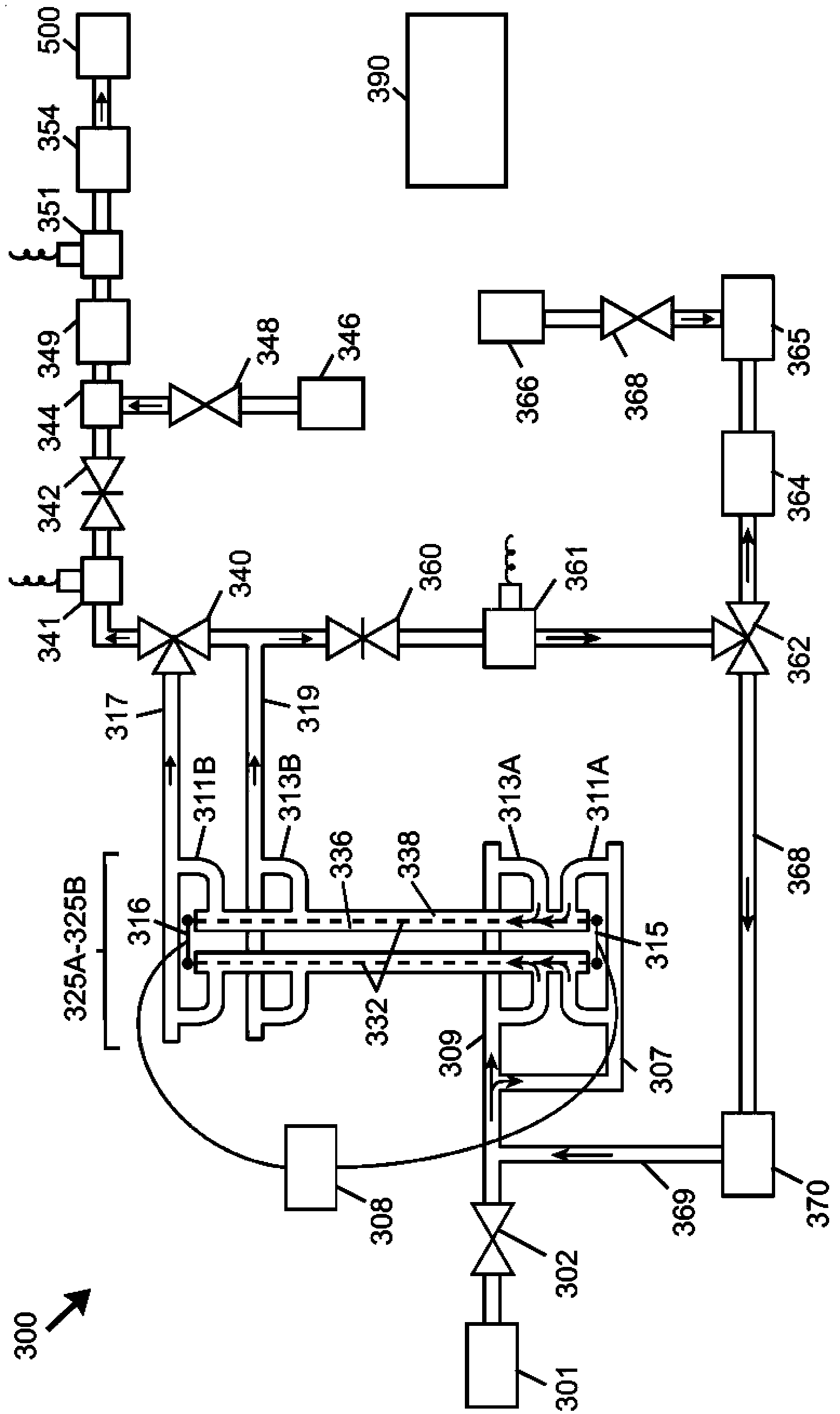
Electrochemical generation of carbon dioxide and hydrogen from organic acids
InactiveUS6387228B1Dissolution rateControllably operatedCellsPressure infusionOrganic acidElectricity
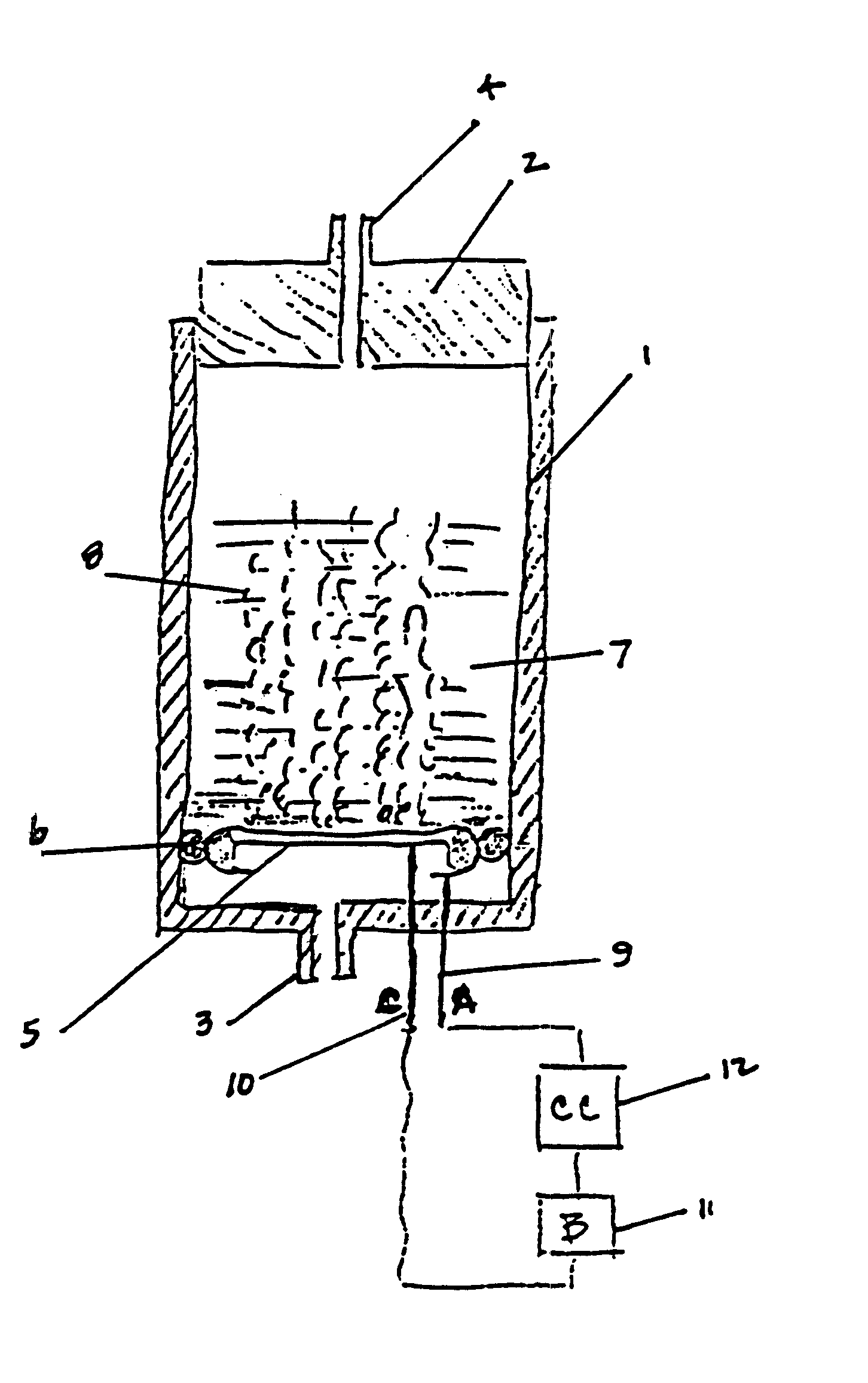
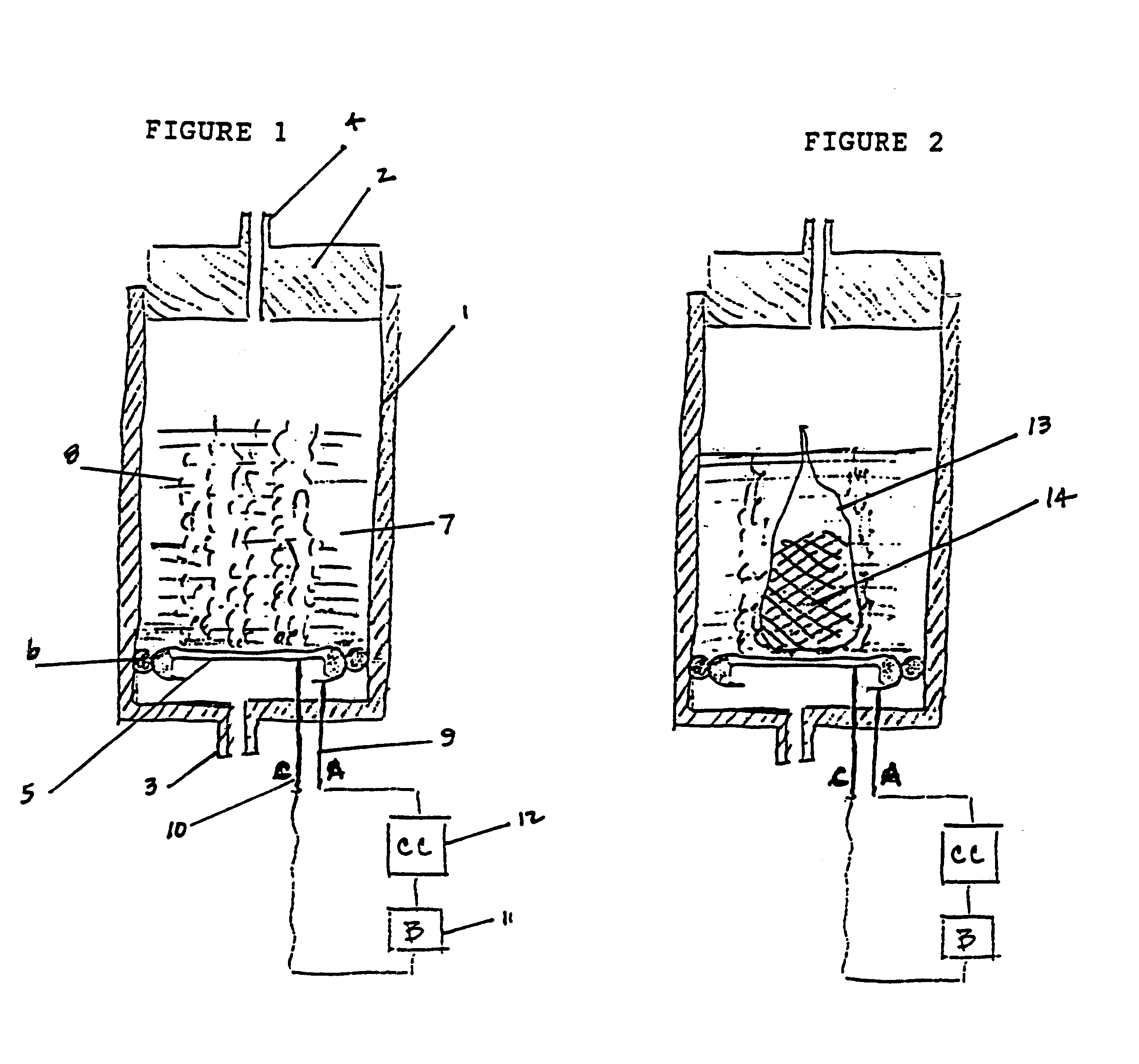
A device as described for the generation of high purity carbon dioxide (CO2) and hydrogen (H2) by electrochemical decomposition of aqueous solutions of liquid and solid organic acids. A d.c. power source is used to apply a preselected current to an electrochemical cell, consisting of an ion permeable membrane and two electrodes. The generation rate of CO2 and H2 is continuous and proportional to the applied current; it can be stopped instantaneously by interrupting the current. Small Battery operated generators can produce propellant CO2 and H2 to deliver fluids from containers. Other uses include the creation of anaerobic environments in incubation chambers.
Owner:M & R CONSULTING SERVICES
Arrangement of ion exchange material within an electrodeionization apparatus
An electrodeionization apparatus is provided comprising an ion-concentrating compartment partially bounded by an anion permeable membrane and also partially bounded by a cation permeable membrane, and a first ion exchange material domain disposed within the ion-concentrating compartment, wherein the first ion exchange material domain is contiguous with at least a portion of an ion-concentrating compartment side surface of one of the anion permeable membrane and the cation permeable membrane, and is spaced apart from the other one of the one of the anion permeable membrane and the cation permeable membrane. In the case where the one of the anion permeable membrane and the cation permeable membrane, having the at least a portion of an ion-concentrating compartment side surface with which the first ion exchange material domain is contiguous, is an anion permeable membrane, the first ion exchange material domain is an anion exchange material predominant domain. In the case where the one of the anion permeable membrane and the cation permeable membrane, having the at least a portion of an ion-concentrating compartment side surface with which the first ion exchange material domain is contiguous, is a cation permeable membrane, the first ion exchange material domain is a cation exchange material predominant domain.
Owner:BL TECH INC
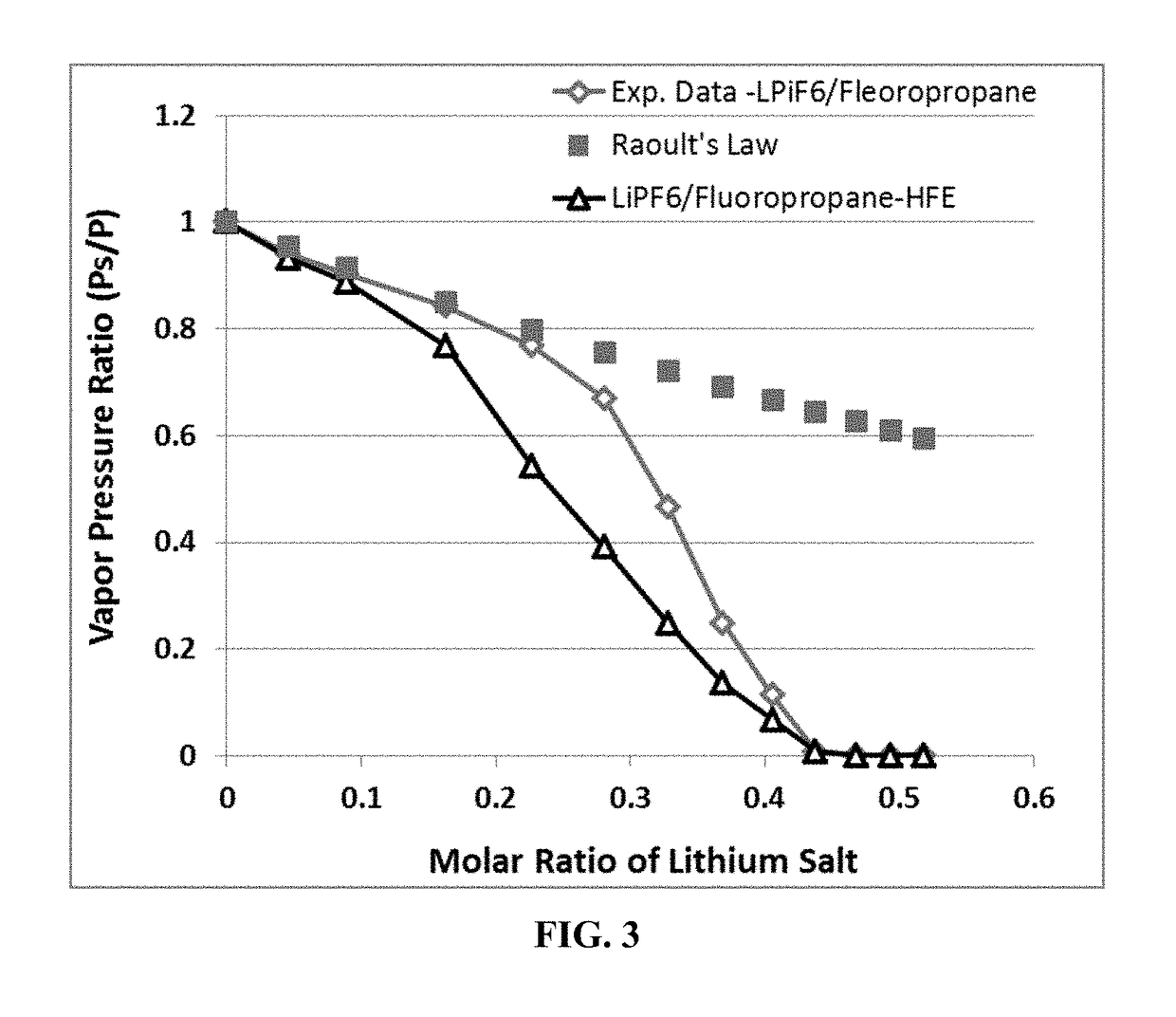
Non-flammable Electrolyte Containing Liquefied Gas and Lithium Secondary Batteries Containing Same
ActiveUS20180375156A1Flammability of any liquefied gas solvent can be effectively suppressedImprove solubilityOrganic chemistryCell electrodesElectrolytic agentDifluoroethyne
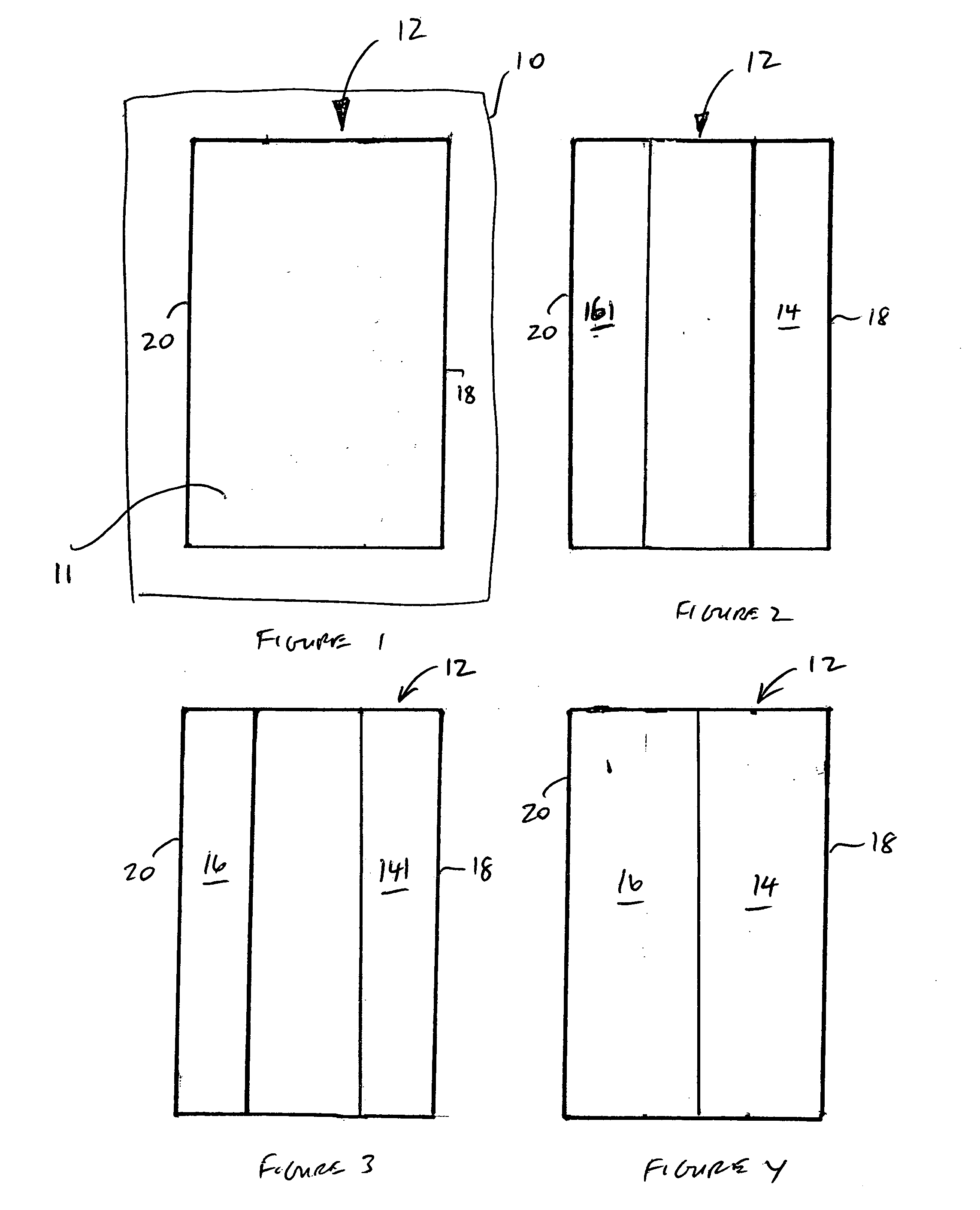
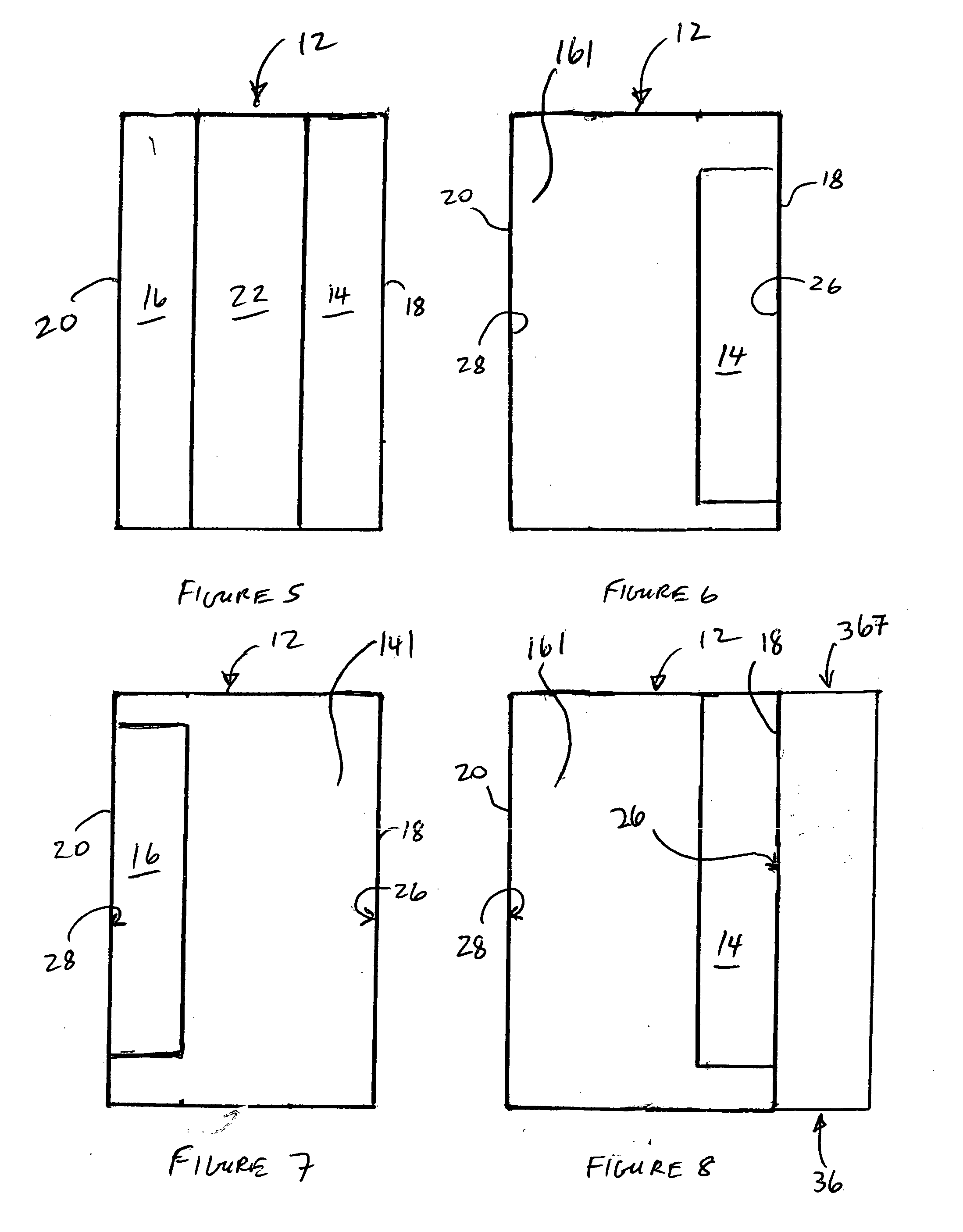
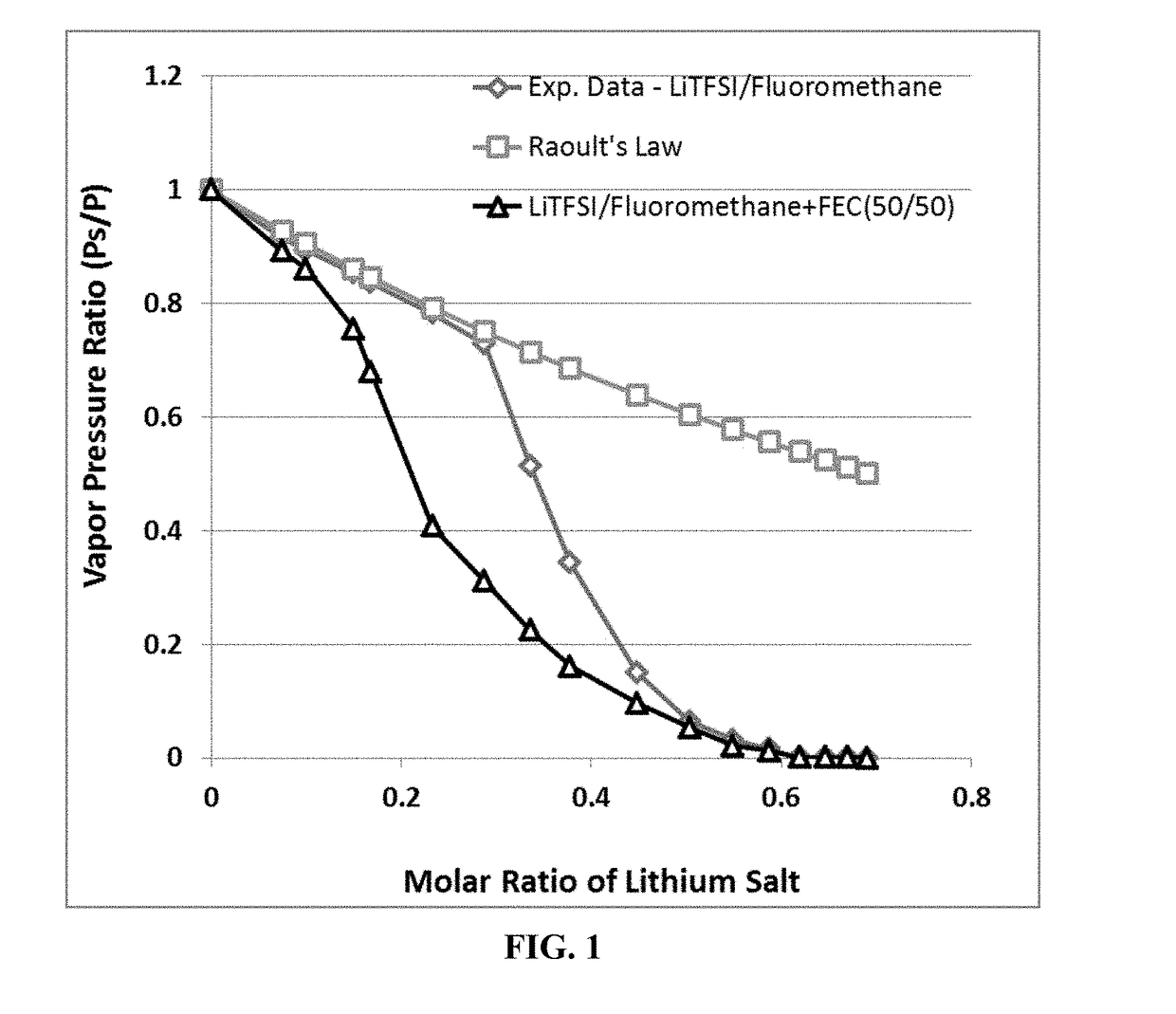
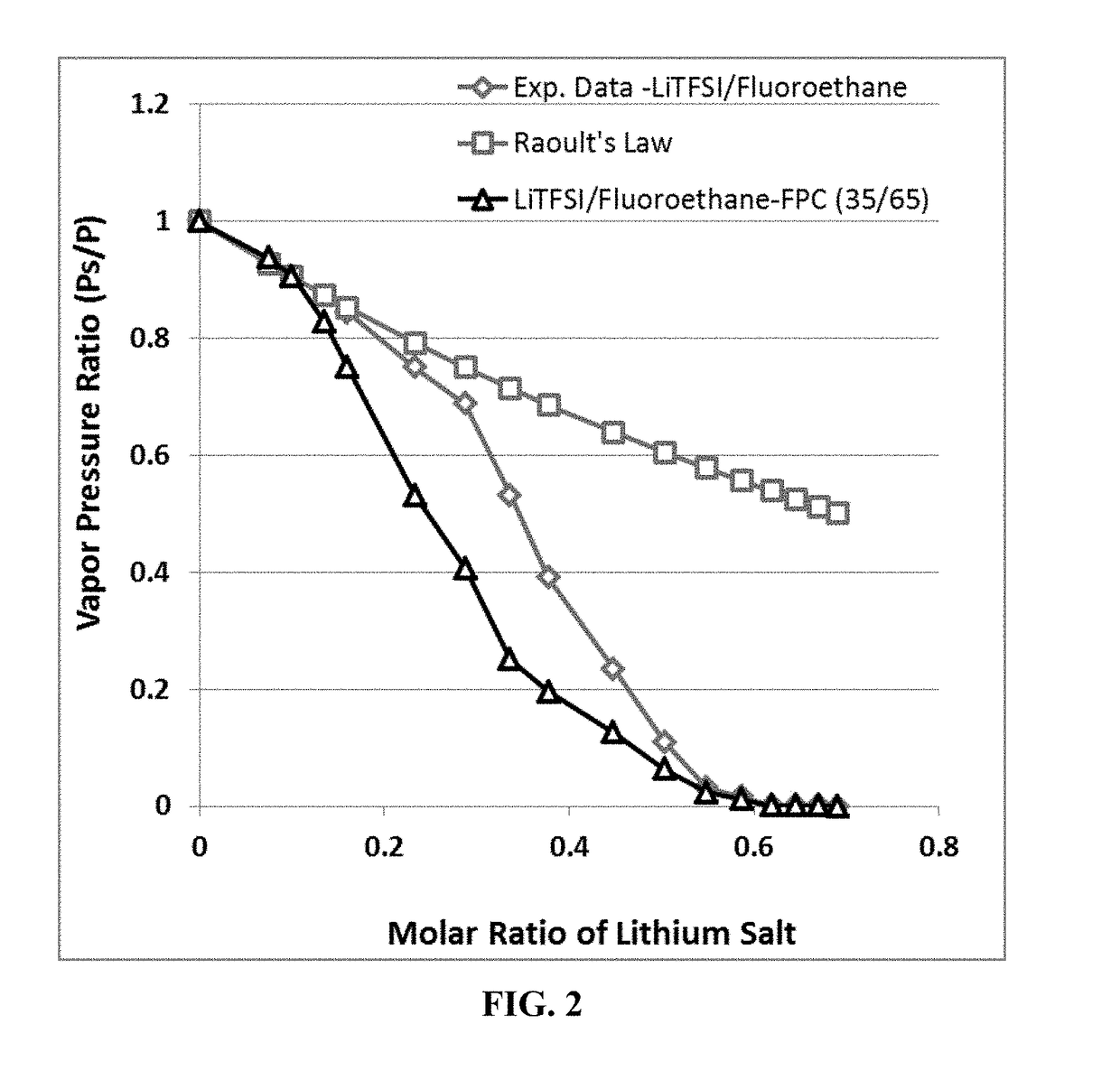
A rechargeable lithium cell comprising a cathode, an anode, an optional ion-permeable membrane disposed between the anode and the cathode, a non-flammable salt-retained liquefied gas electrolyte in contact with the cathode and the anode, wherein the electrolyte contains a lithium salt dissolved in or mixed with a liquefied gas solvent having a lithium salt concentration greater than 1.0 M so that the electrolyte exhibits a vapor pressure less than 1 kPa when measured at 20° C., a vapor pressure less than 60% of the vapor pressure of the liquefied gas solvent alone, a flash point at least 20 degrees Celsius higher than a flash point of the liquefied gas solvent alone, a flash point higher than 150° C., or no flash point, wherein the liquefied gas solvent is selected from methane, fluoromethane, difluoromethane, chloromethane, dichloromethane, ethane, fluoroethane, difluoroethane, tetrafluoroethane, chloroethane, dichloroethane, tetrachloroethane, propane, fluoropropane, chloropropane, ethylene, fluoroethylene, chloroethylene, or a combination thereof.
Owner:GLOBAL GRAPHENE GRP INC
Method of generating electrolyzed water and electrolyzed water generation apparatus therefor
InactiveUS20080047844A1Easy to carryCellsWater treatment parameter controlElectrolysed waterGeneral family
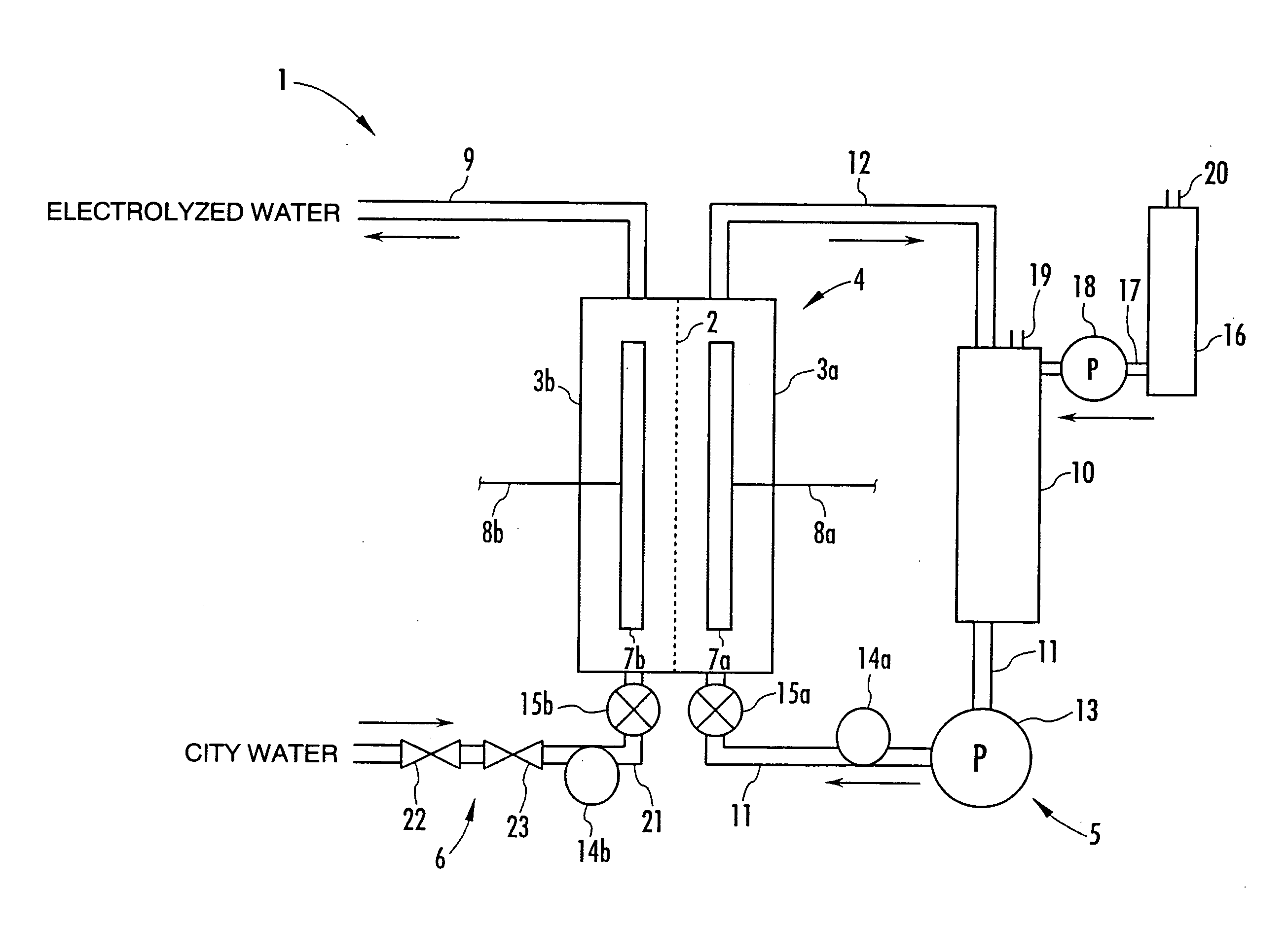
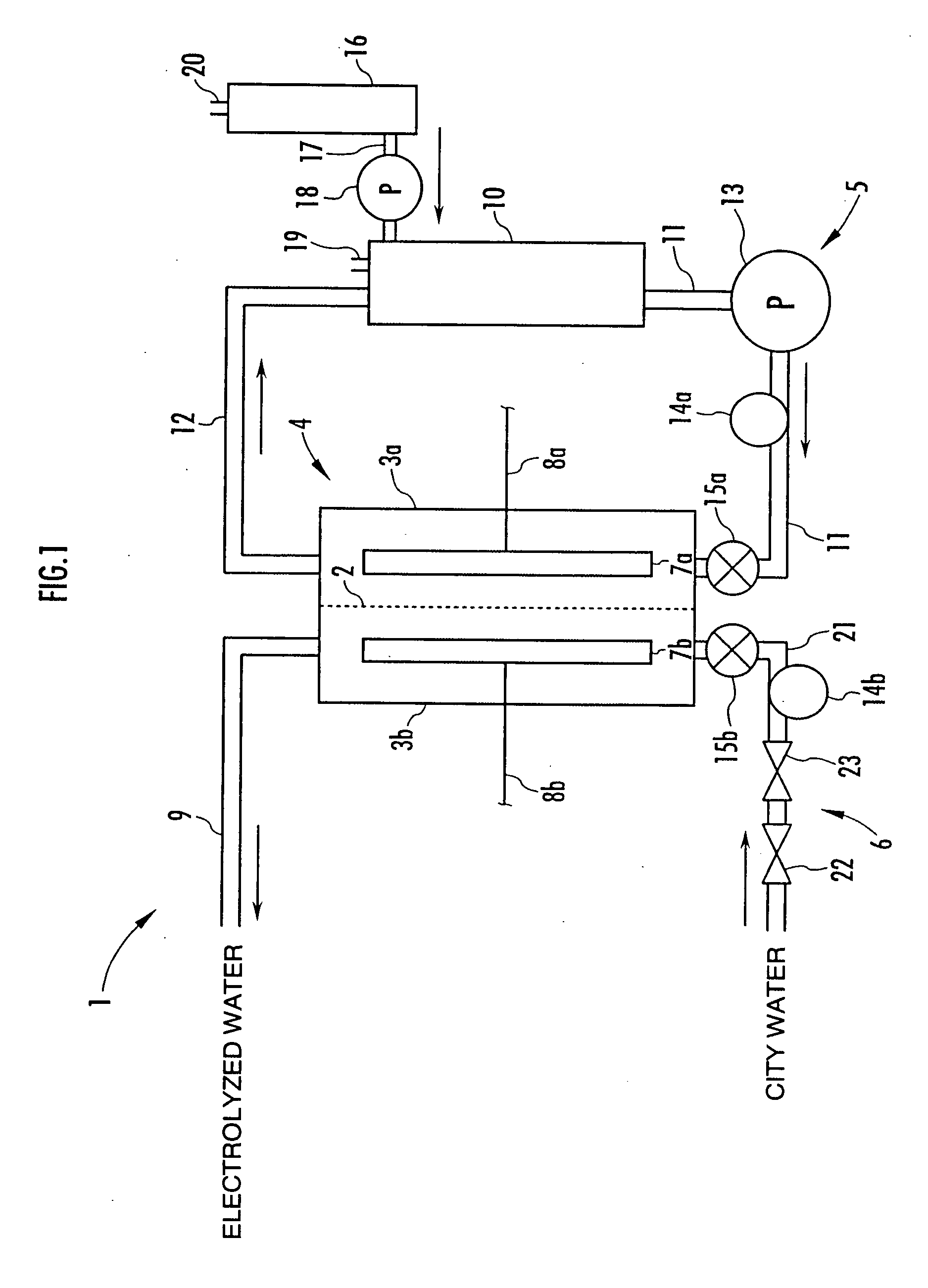
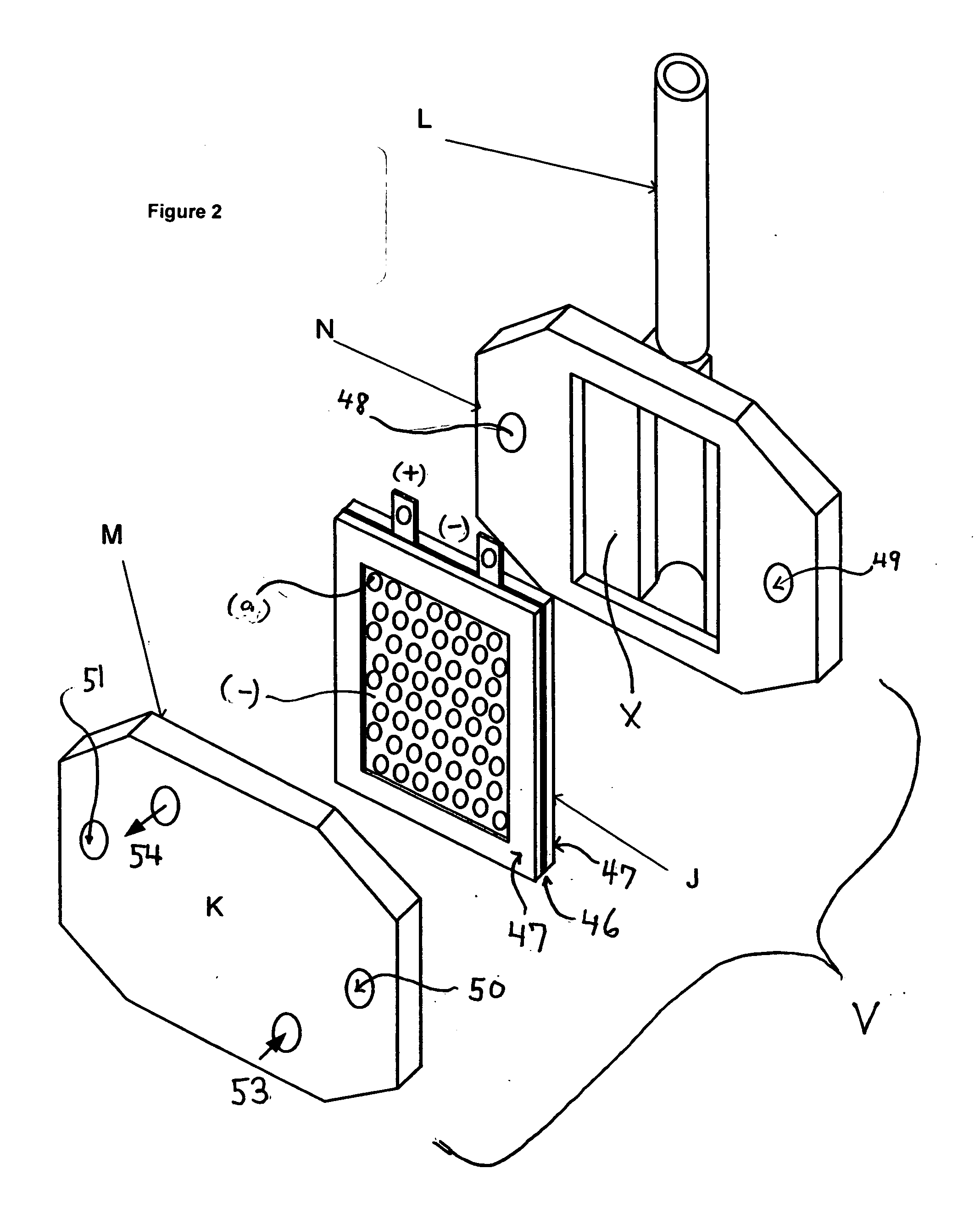
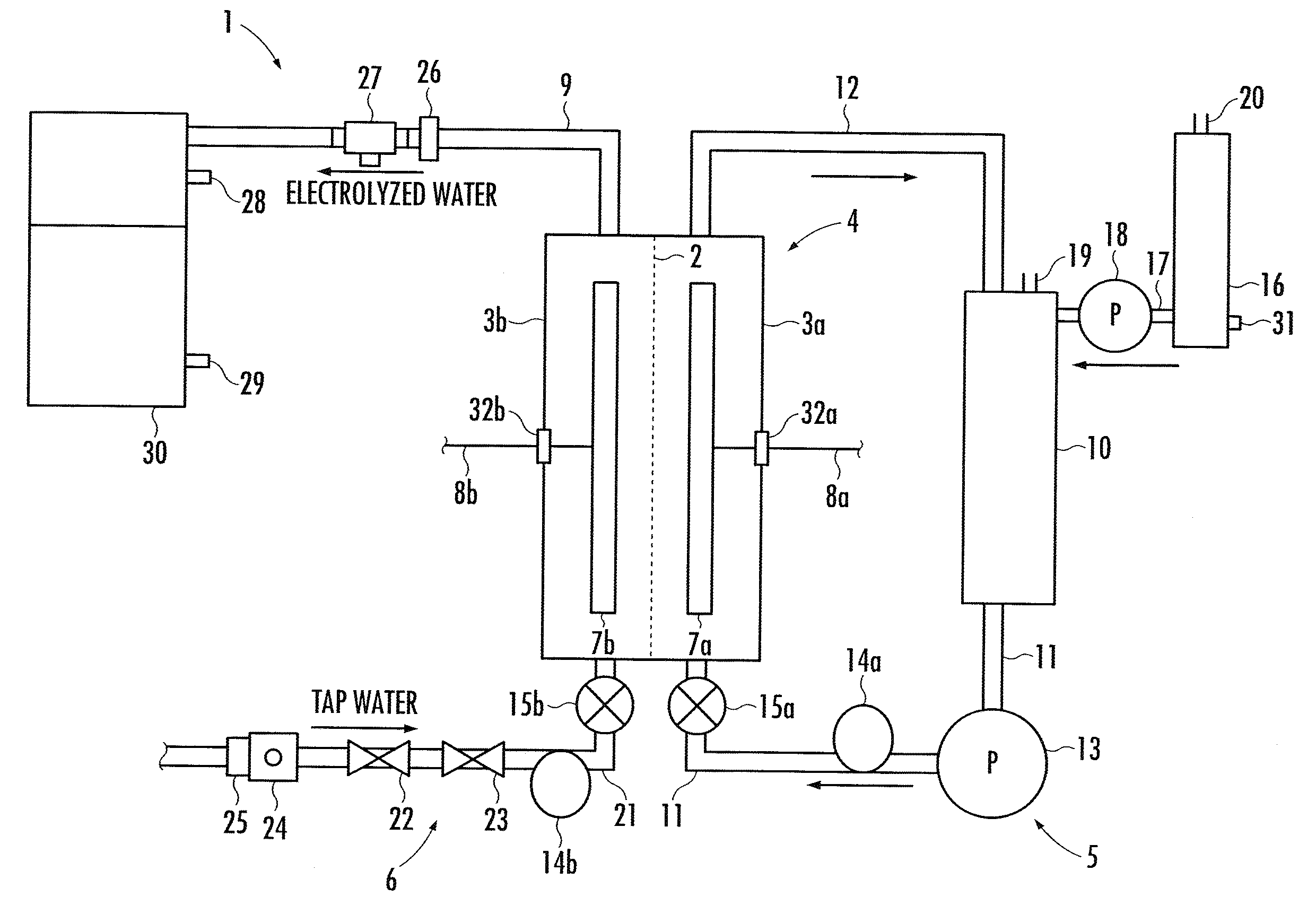
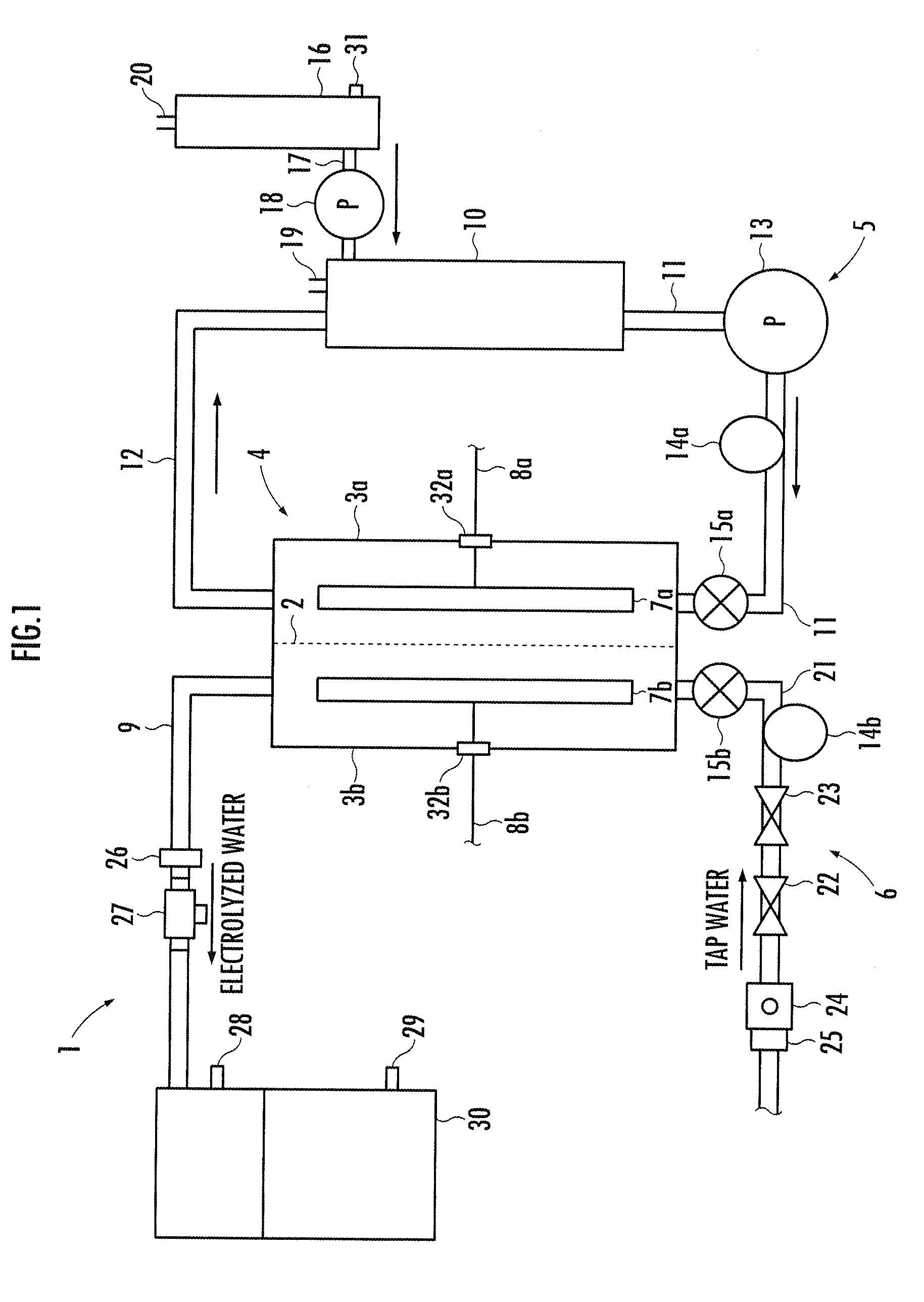
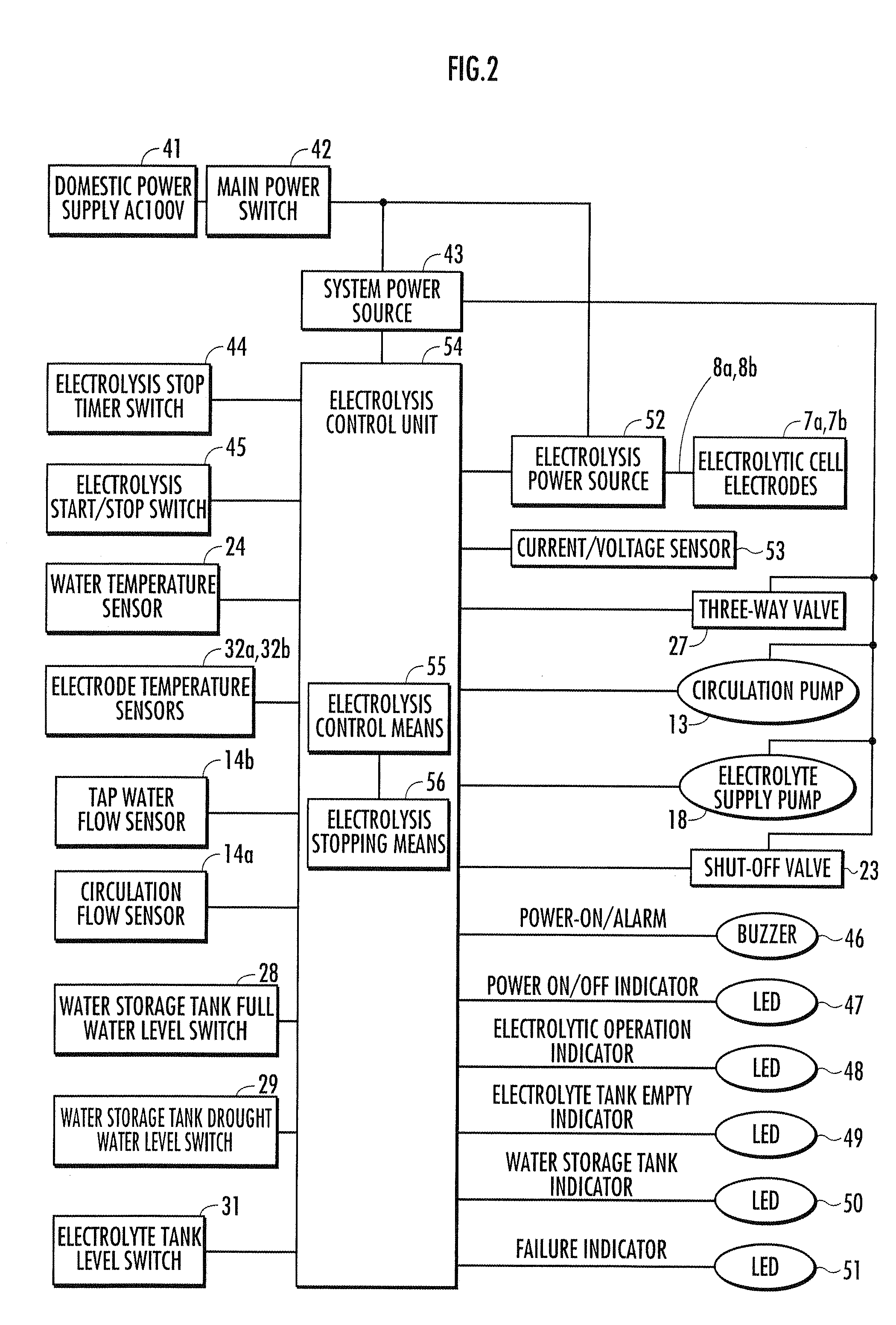
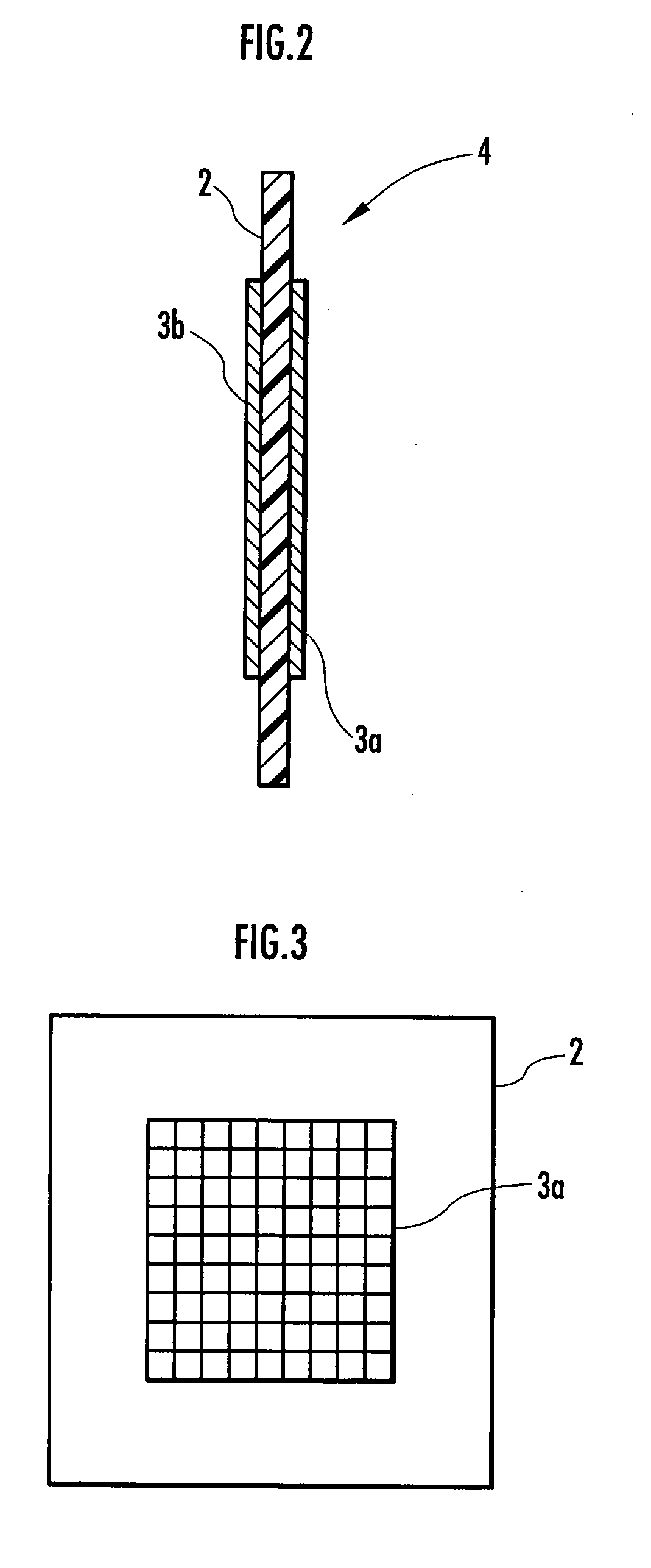
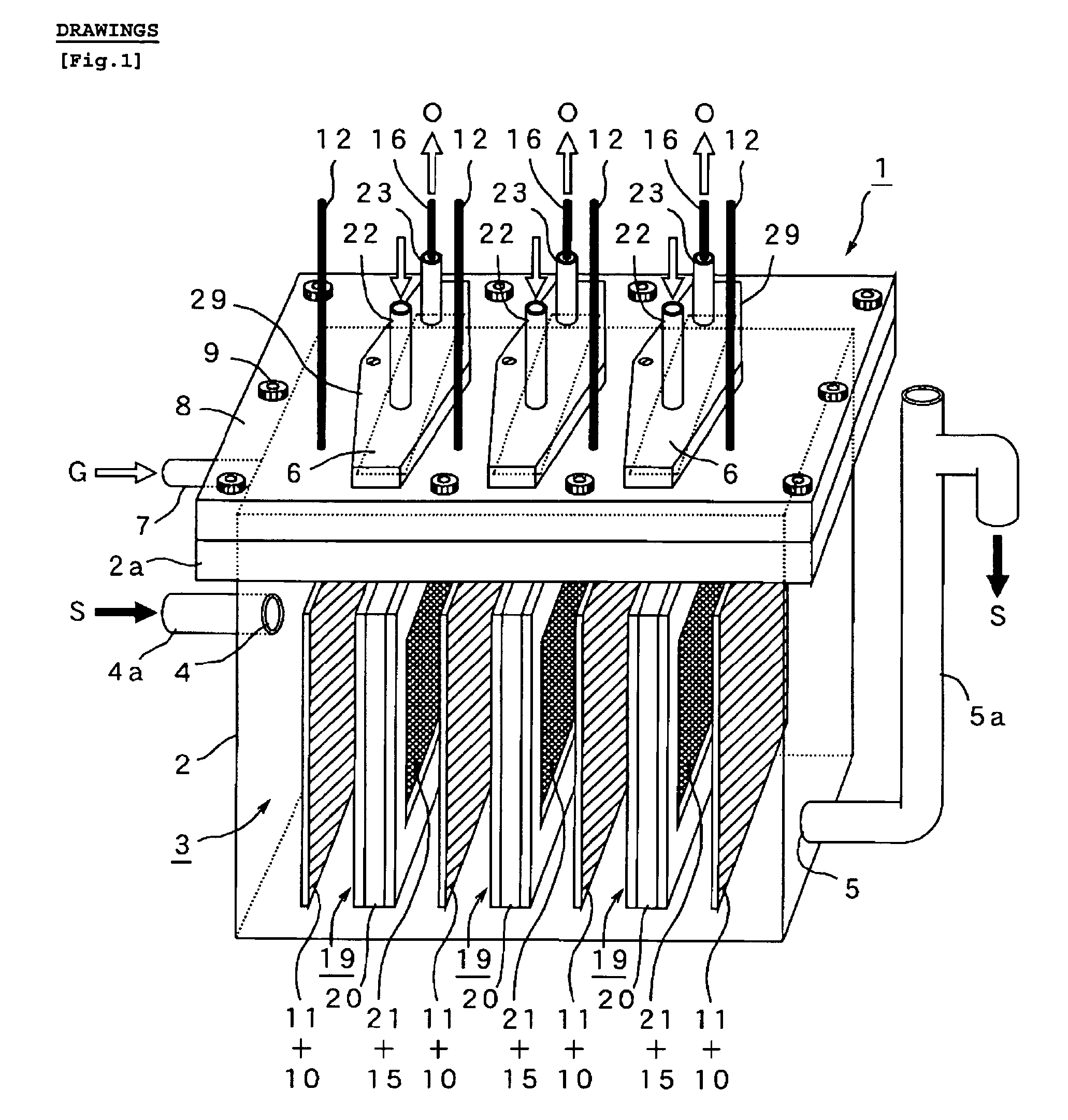
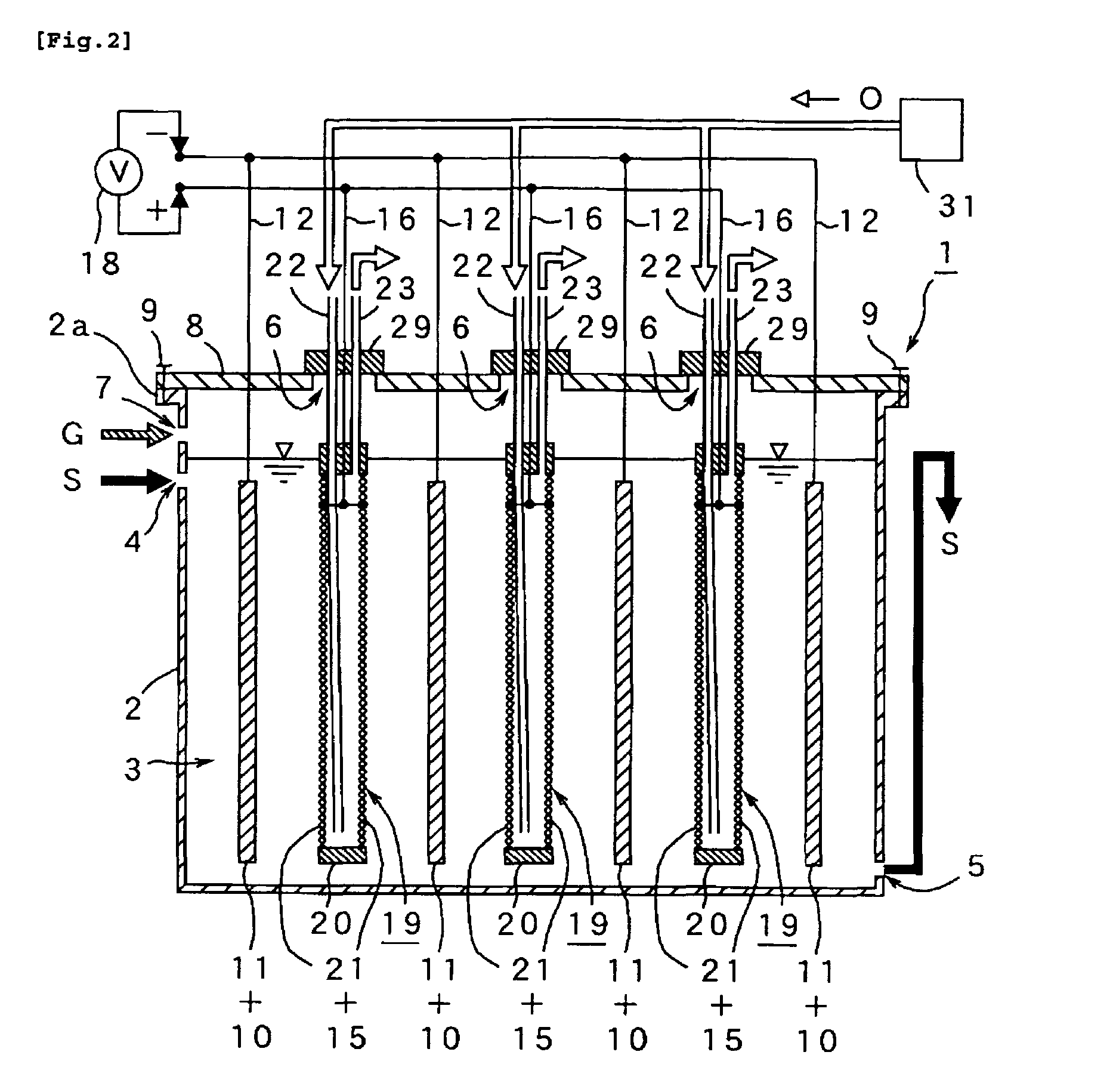
A method and apparatus for generating electrolyzed water easy to carry out or handle even in ordinary homes. An electrolyte aqueous solution is circuited through a first electrolysis chamber 3a of two electrolysis chambers placed on opposite sides of an ion-permeable membrane 2, and raw water is supplied only to the second electrolysis chamber 3b. A voltage is applied between electrodes 7a and 7b to cause electrolysis. Electrolyzed water generated in the second electrolysis chamber 3b is drawn out. The concentration of the electrolyte aqueous solution circulated through the first electrolysis chamber 3a is maintained within a predetermined range. The membrane 2 is an anion-exchange membrane. The electrolyte aqueous solution is circulated through the first cathode-side electrolysis chamber 3a; raw water is supplied only to the second anode-side electrolysis chamber 3b; and acid electrolyzed water generated in the anode-side electrolysis chamber 3a is drawn out. The electrolyte aqueous solution is NaCl or KCl aqueous solution. The concentration is maintained within the predetermined range by adding hydrochloric acid according to the pH of the NaCl or KCl solution or the amount of reaction of this solution computed from the amount of energization during the electrolysis.
Owner:HONDA MOTOR CO LTD
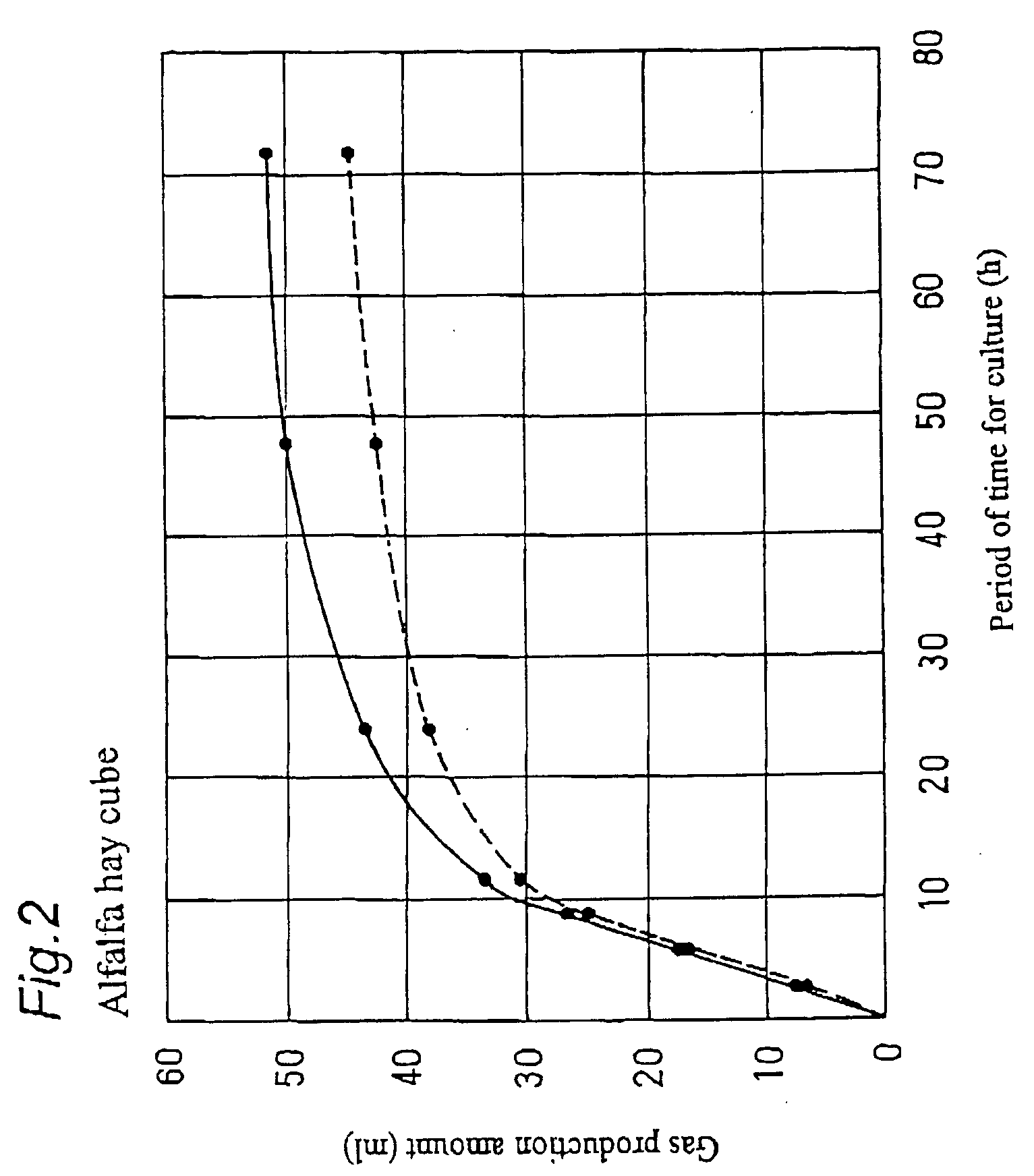
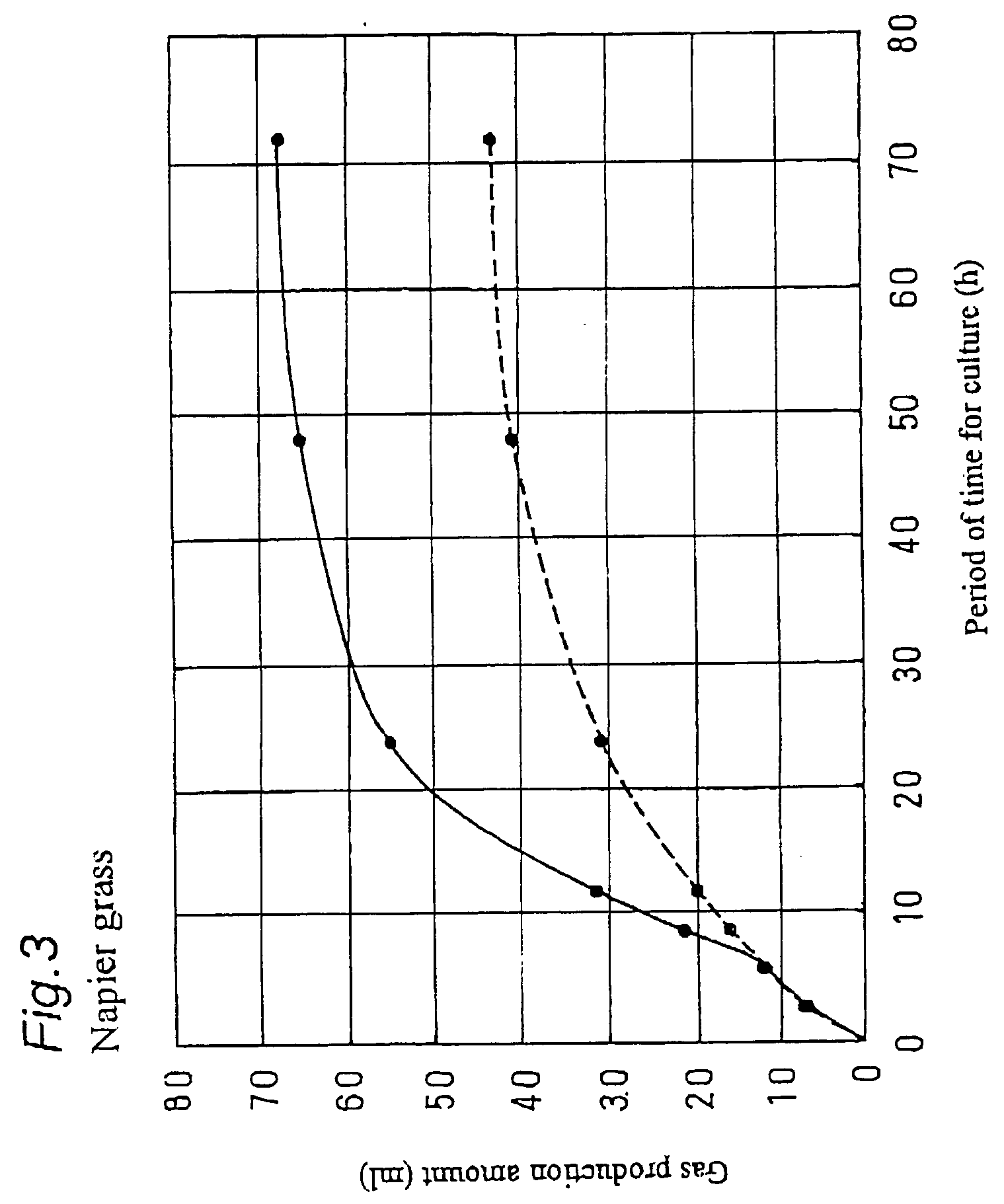
Digestion promoter for ruminant animal and breeding method of ruminant animal
A digestion promoter for a ruminant animal for promoting digestion of feed in a stomach of the ruminant animal, containing, as a main component, electrolyzed weak alkaline water produced in an electrolytic cell with an ion-permeable membrane. A breeding method of a ruminant animal, providing plants as feed for the ruminant animal with drinking water containing, as a main component, electrolyzed weak alkaline water produced in an electrolytic cell with an ion-permeable membrane.
Owner:HOSHIZAKI ELECTRIC CO LTD
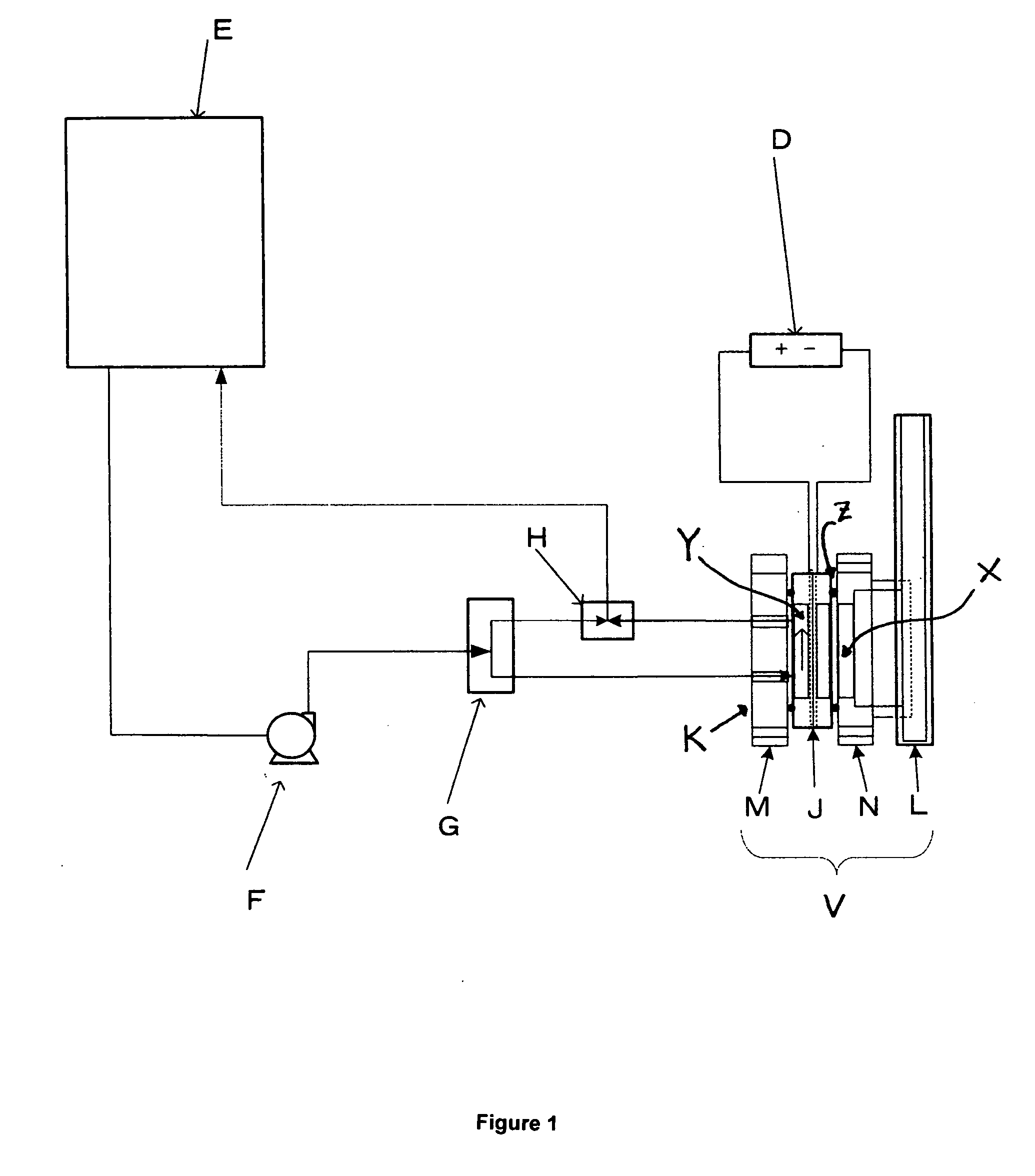
Acidic electrolyzed water production system and generation control method
ActiveUS20060260954A1Good removal effectEasy to disassembleCellsWater/sewage treatment apparatusTectorial membraneElectrolysis
An improved device and method for the creation of acidic electrolyzed water is described. The device has an flow-through anode chamber and a static cathode chamber. The static cathode chamber contains a fixed amount of salt-containing electrolyte, which is renewed as needed. The flow rate of water through the anode is restricted to a range of about 5 to 40 ml per ampere of current passed through the electrode. Electrolyzed water flowing from the anode is diluted to obtain the desired concentration of hypochlorous acid, and is collected in a tank or other vessel. The electrolysis reaction is terminated when a preset amount of current has passed through the anode. Water circulation may be one pass or recycling. In a preferred embodiment, the membrane is anion-selective. Preferably, the membrane and the electrodes are integrated into a preassembled format that can be attached to the anode and cathode compartments via flanges or similar devices allowing quick replacement of an electrode assembly in an electrolyzer. The anion-permeable membrane can be protected by a protection membrane, in which are provided slits or other discontinuities to allow venting of gas.
Owner:ECOLAB USA INC
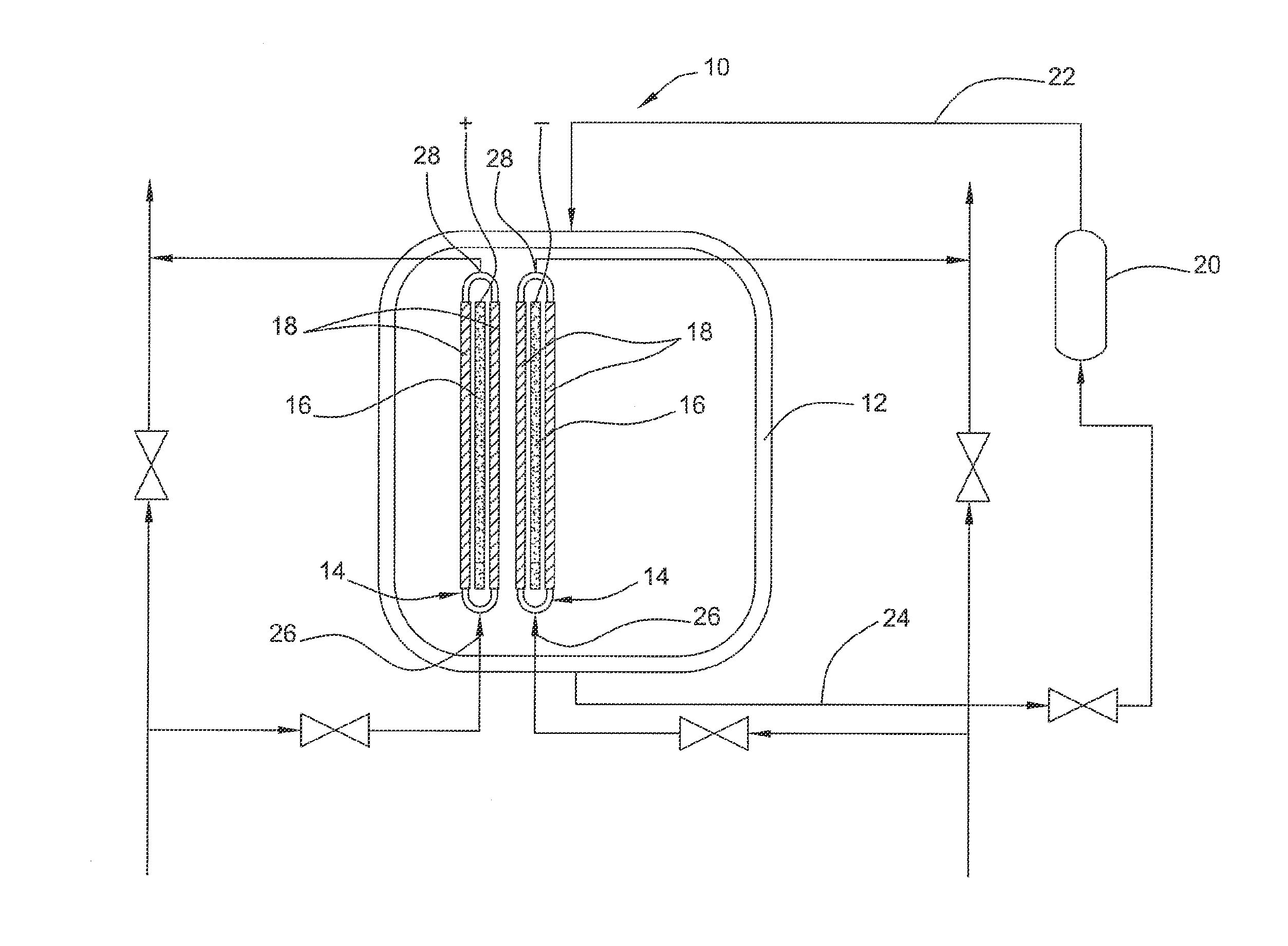
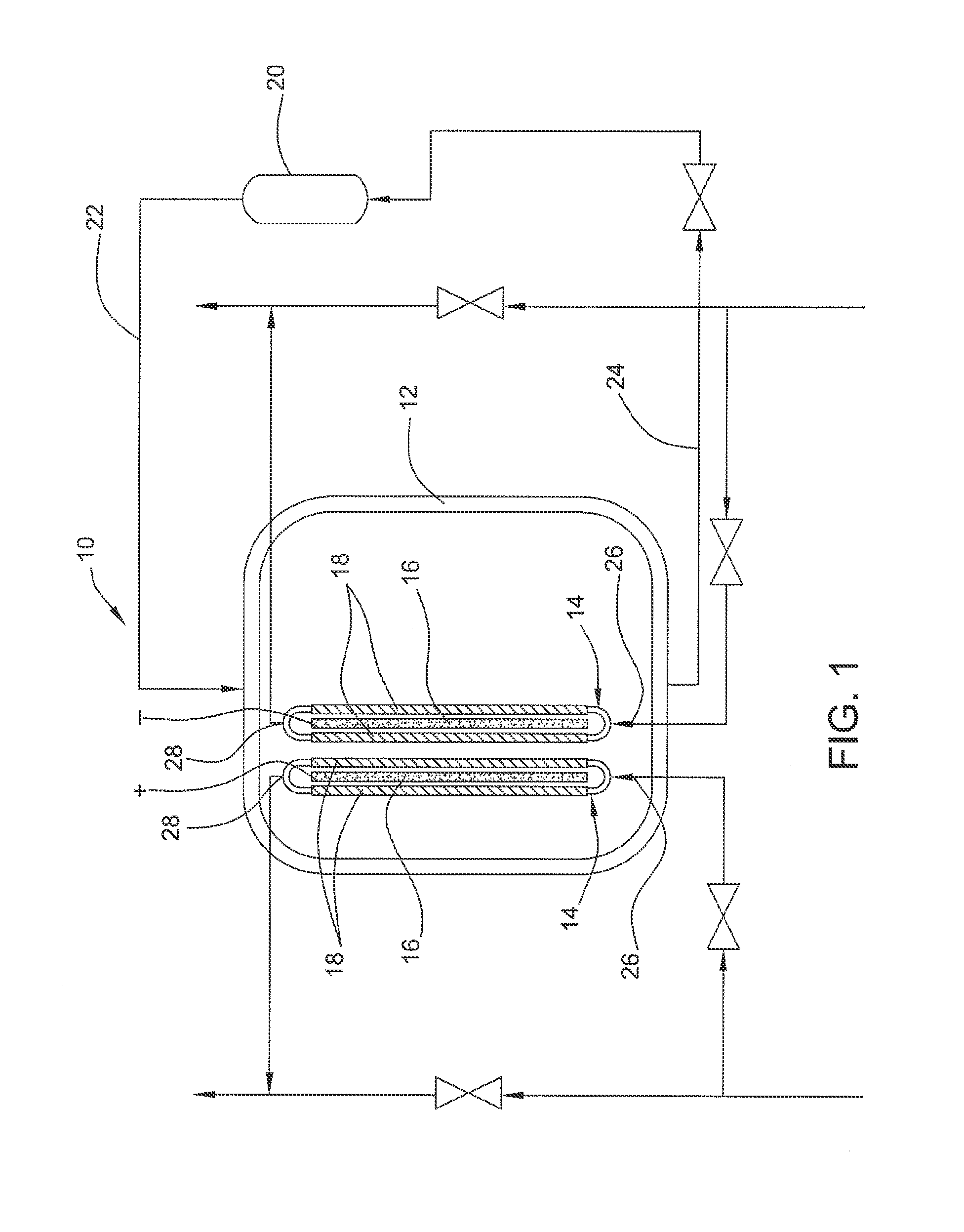
Electrolyzed water producing method and apparatus
InactiveUS20090071843A1Low costSmall sizeCellsWater treatment parameter controlElectrolysed waterAqueous electrolyte
Electrolyzed water producing method and apparatus are provided which are capable of producing electrolyzed water having a desired property irrespective of the quality of raw water supplied and the like while allowing the size and weight of the apparatus and the cost to be reduced by limiting the capacity of an electrolysis power source. The electrolyzed water producing method includes: circulating an aqueous electrolyte solution to a first electrolytic chamber of a pair of electrolytic chambers opposed to each other across an intervening ion permeable diaphragm while supplying raw water to the second electrolytic chamber; and applying a predetermined voltage to a pair of electrodes disposed in the respective electrolytic chambers with the diaphragm intervening there between, to electrolyze the raw water and the aqueous electrolyte solution, thereby producing electrolyzed water in the second electrolytic chamber.
Owner:HONDA MOTOR CO LTD
Electrochemical generation of carbon dioxide and hydrogen from organic acids
InactiveUS6780304B1Controllably operatedReduce processing stepsCellsPressure infusionElectricityOrganic acid
A device as described for the generation of high purity carbon dioxide (CO2) and hydrogen (H2) by electrochemical decomposition of aqueous solutions of liquid and solid organic acids. A d.c. power source is used to apply a pre-selected current to an electrochemical cell, consisting of an ion permeable membrane and two electrodes. The generation rate of CO2 and H2 are continuous and proportional to the applied current; it can be stopped instantaneously by interrupting the current. Small battery operated generators can produce propellant CO2 and H2 to deliver fluids from containers other uses include the creation of anaerobic environments in incubation chambers.
Owner:MAGET HENRI J R
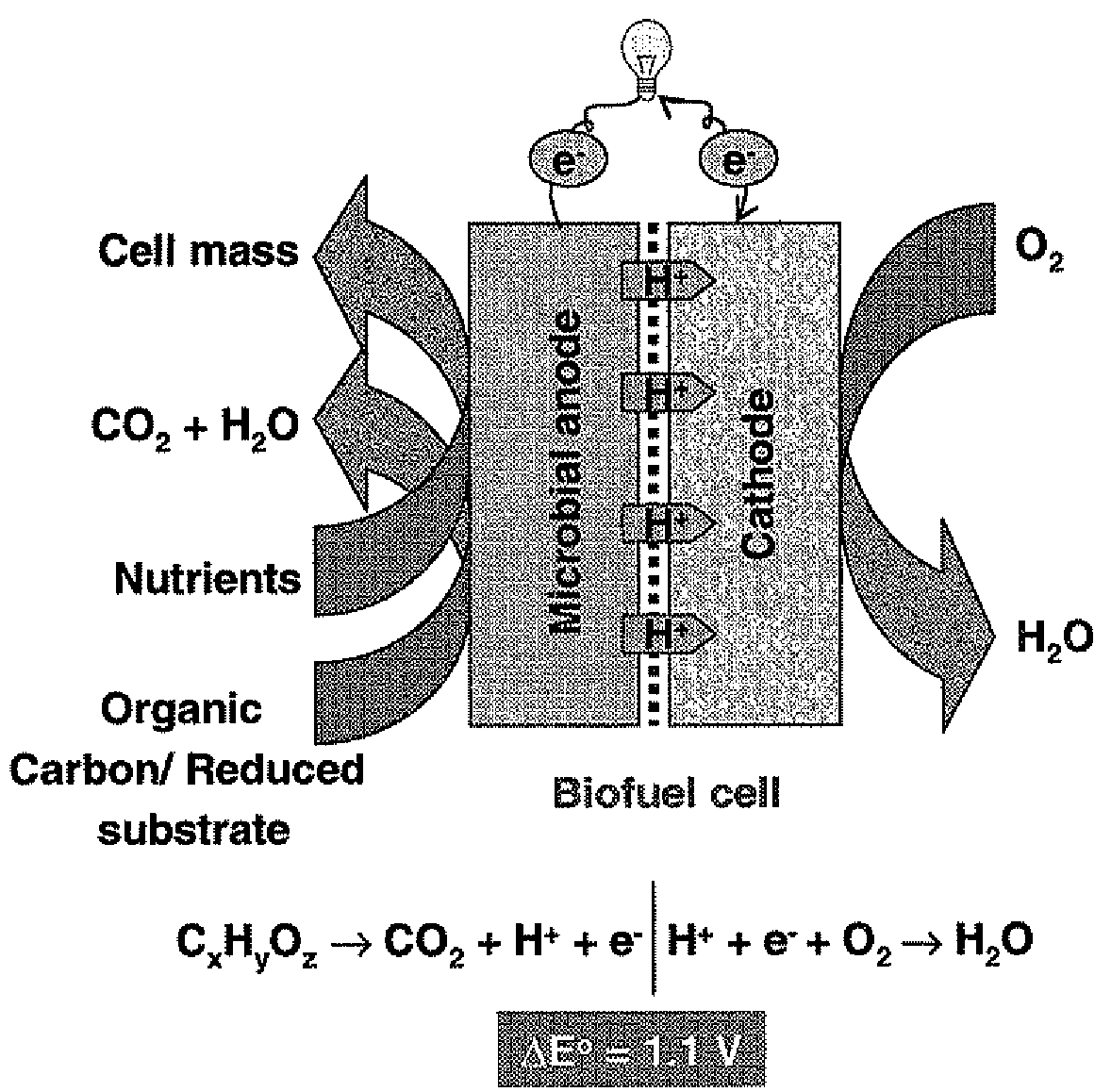
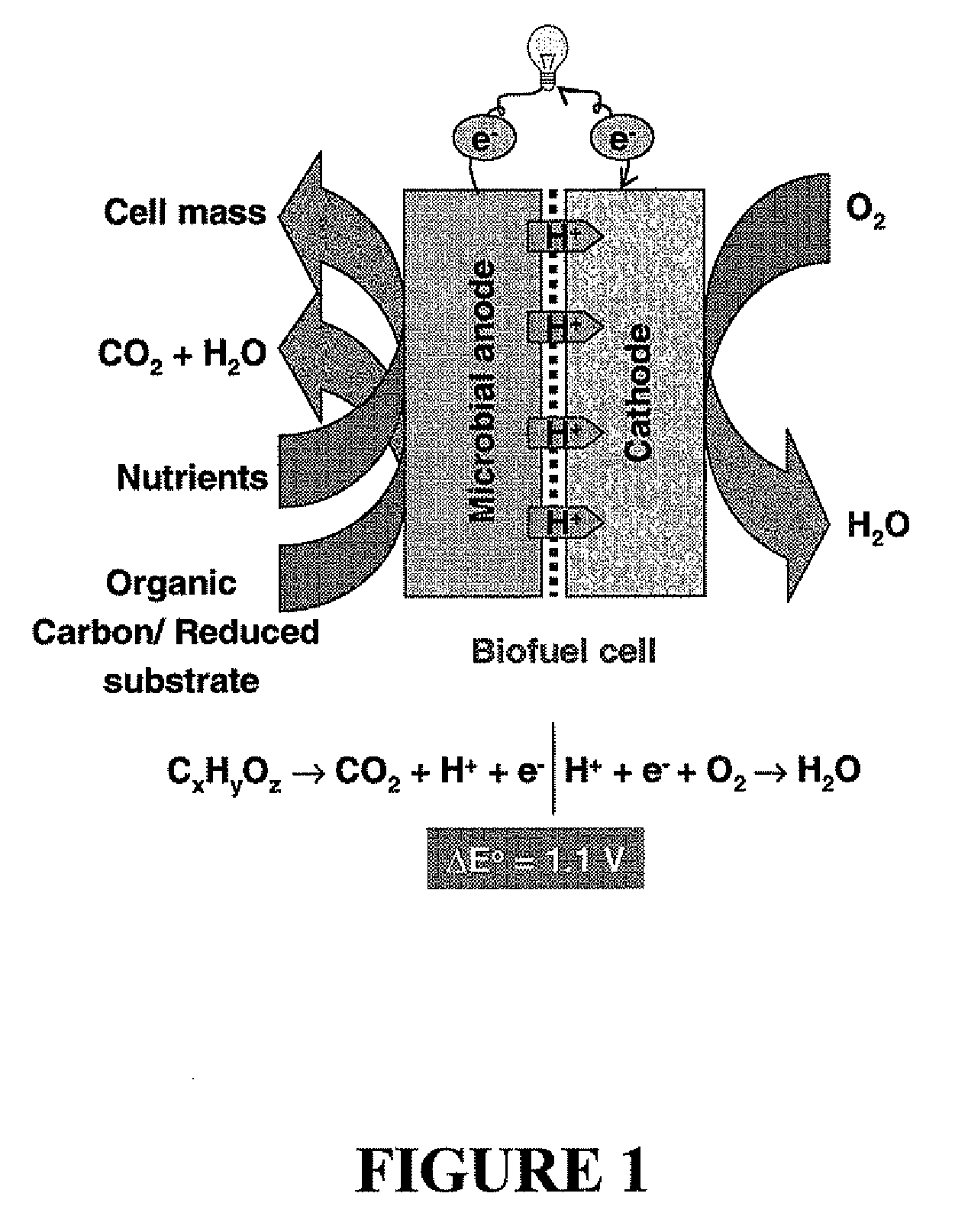
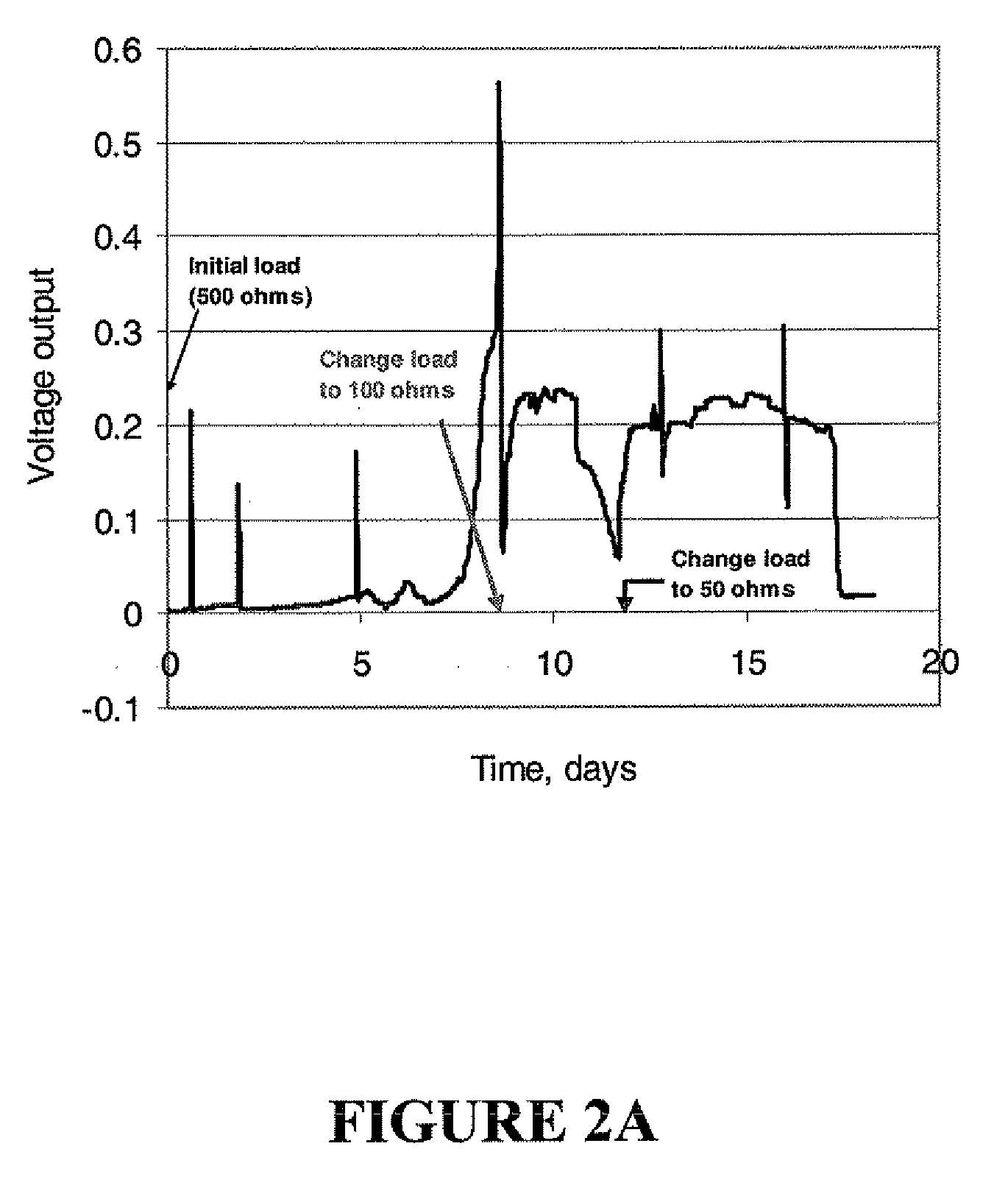
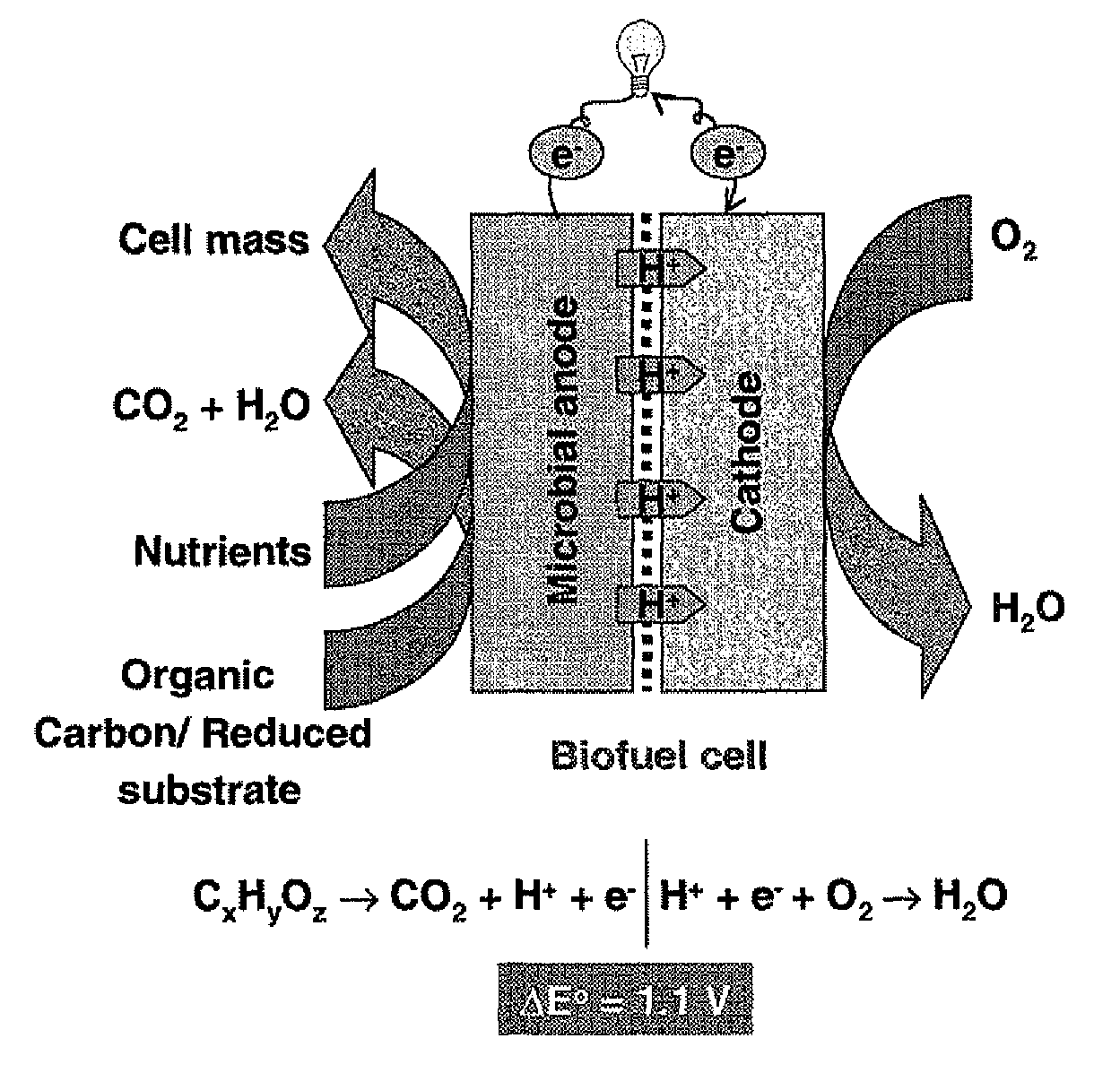
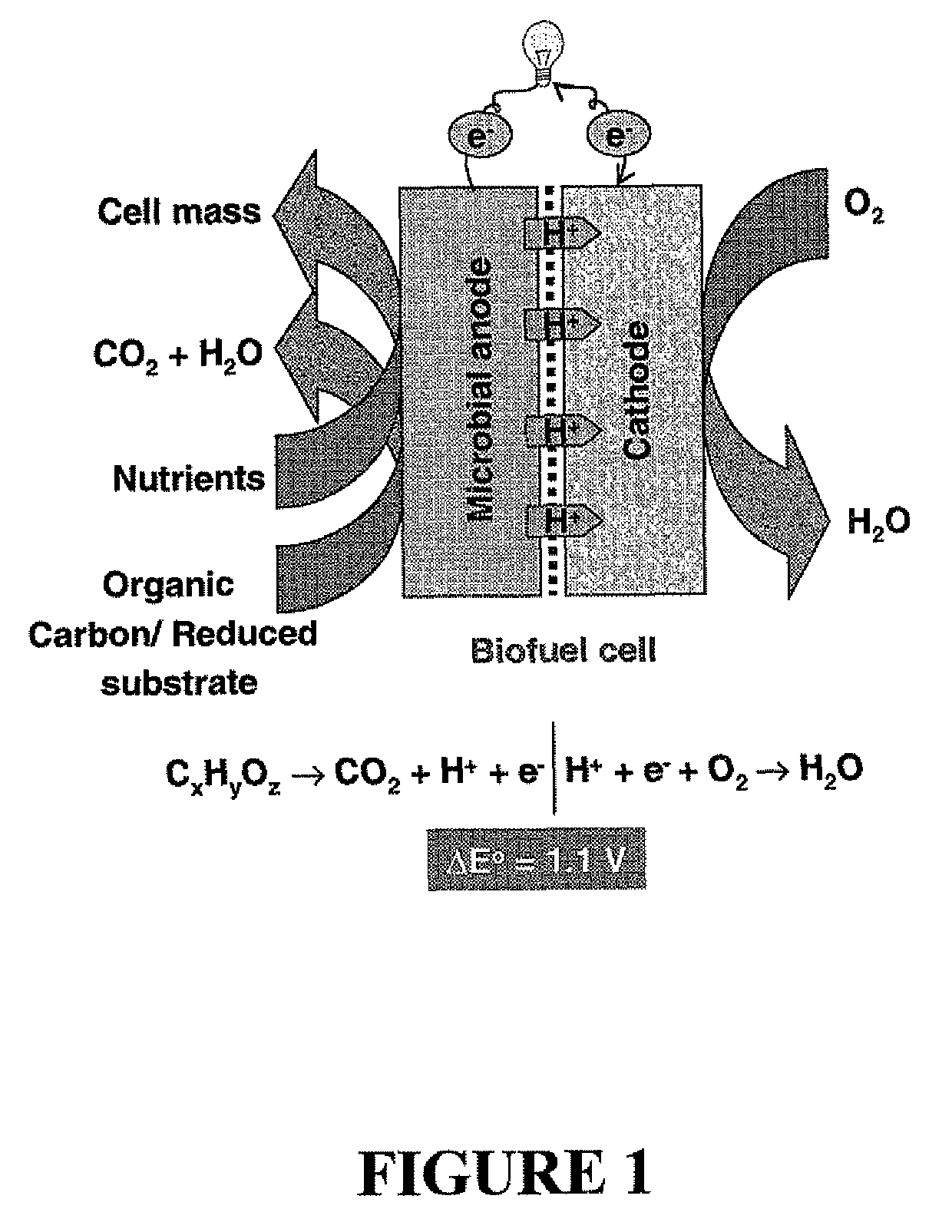
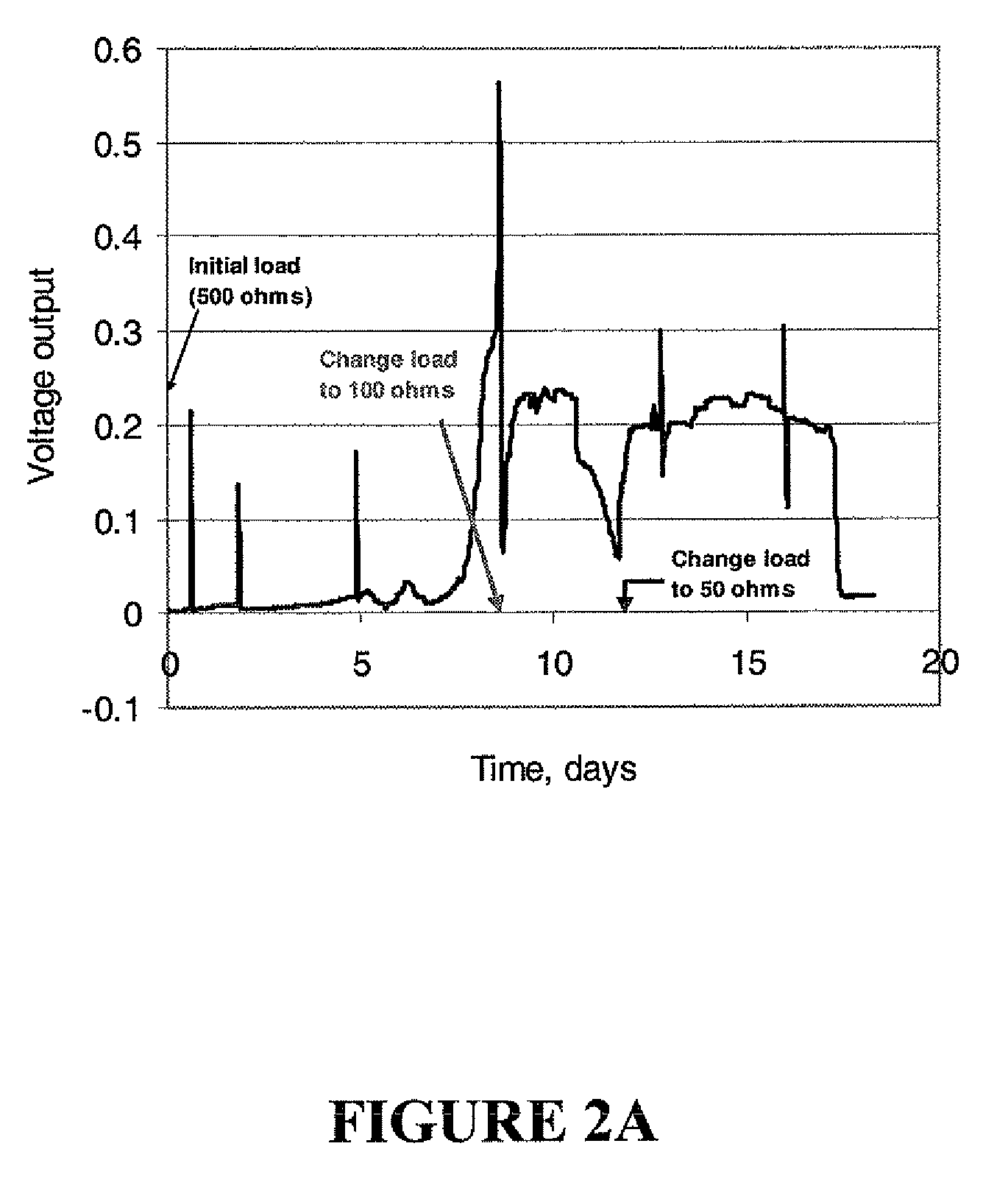
Microbial fuel cell treatment of ethanol fermentation process water
ActiveUS20100203359A1Increase productionEconomical, convenient, and environmentally friendly methodBacteriaBiofuelsMicrobial fuel cellHydrogen
The present invention relates to a method for removing inhibitor compounds from a cellulosic biomass-to-ethanol process which includes a pretreatment step of raw cellulosic biomass material and the production of fermentation process water after production and removal of ethanol from a fermentation step, the method comprising contacting said fermentation process water with an anode of a microbial fuel cell, said anode containing microbes thereon which oxidatively degrade one or more of said inhibitor compounds while producing electrical energy or hydrogen from said oxidative degradation, and wherein said anode is in electrical communication with a cathode, and a porous material (such as a porous or cation-permeable membrane) separates said anode and cathode.
Owner:UT BATTELLE LLC
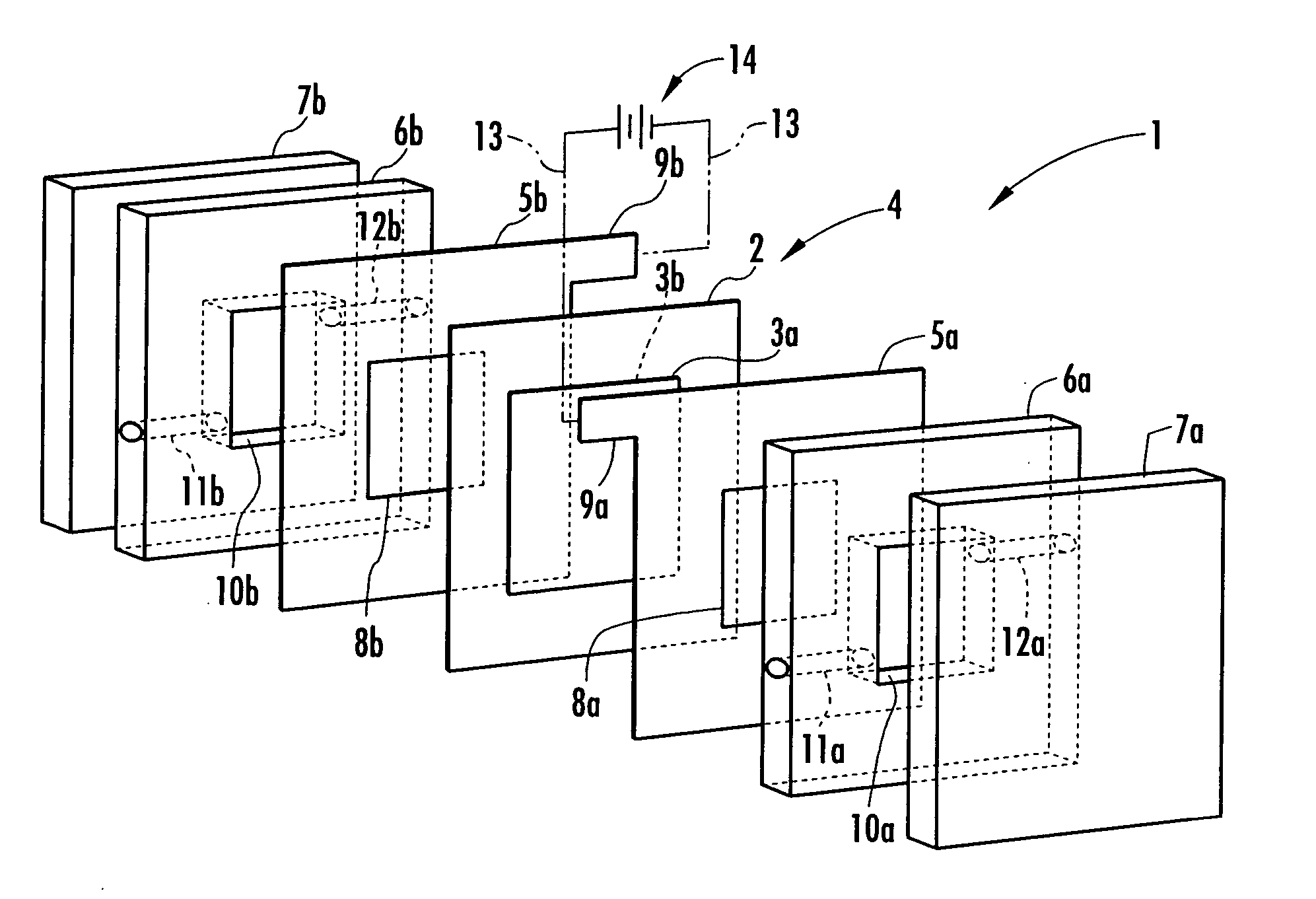
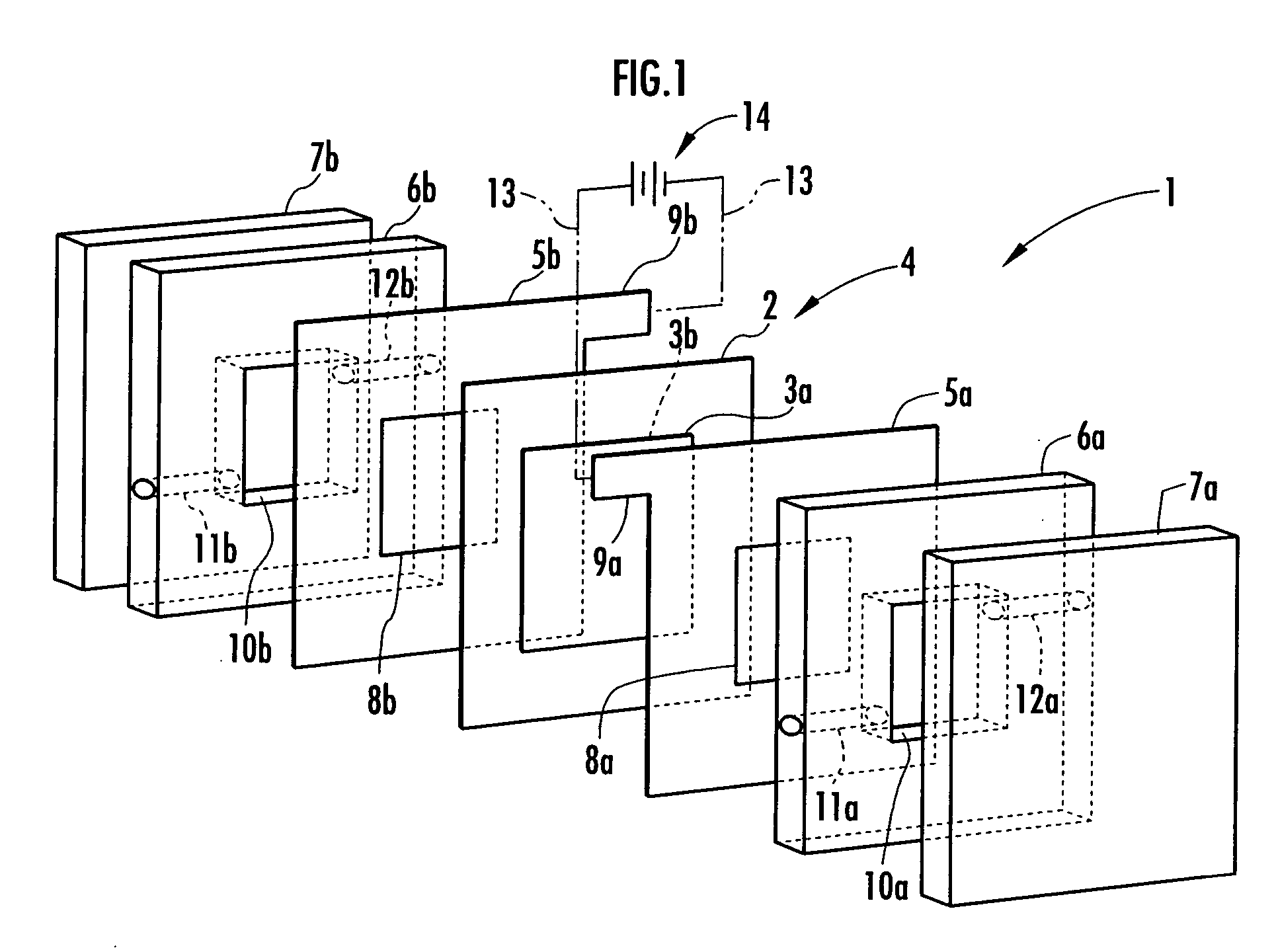
Electrolysis vessel and apparatus for generating electrolyzed water
InactiveUS20070131541A1Reduce weightSimplifies circulation structureCellsWater/sewage treatment apparatusConductive pasteWater discharge
Provided is an electrolysis cell and an electrolyzed water producing equipment which are each small in size, has excellent electrolysis efficiency and can reduce an anion concentration in acidic electrolyzed water. The electrolysis cell is equipped with electrolysis rooms 10a and 10b located opposite to each other via an ion permeable membrane 2, raw water supply units 11a and 11b, electrodes 3a and 3bdisposed with the membrane interposed therebetween, and electrolyzed water discharge units 12a and 12b. The membrane 2 is an anion permeable film. The electrodes 3a and 3b are formed so as to firmly adhere to both surfaces of the anion permeable membrane 2 and expose a portion of the anion permeable membrane 2. Only raw water fed to the electrolysis room 10b on the cathode side contains an electrolyte. The electrodes 3a and 3b are porous and they each has an electrode base material made of a powdery titanium compound such as TiC or TiN, a catalyst such as platinum black or iridium black and a binder such as PVA. The electrodes 3a and 3b may be mesh-shaped or comb-shaped. The electrodes 3a and 3b are formed by applying a conductive paste containing conductive powders onto the surfaces of the anion permeable membrane 2, followed by heating or pressurization.
Owner:HONDA MOTOR CO LTD
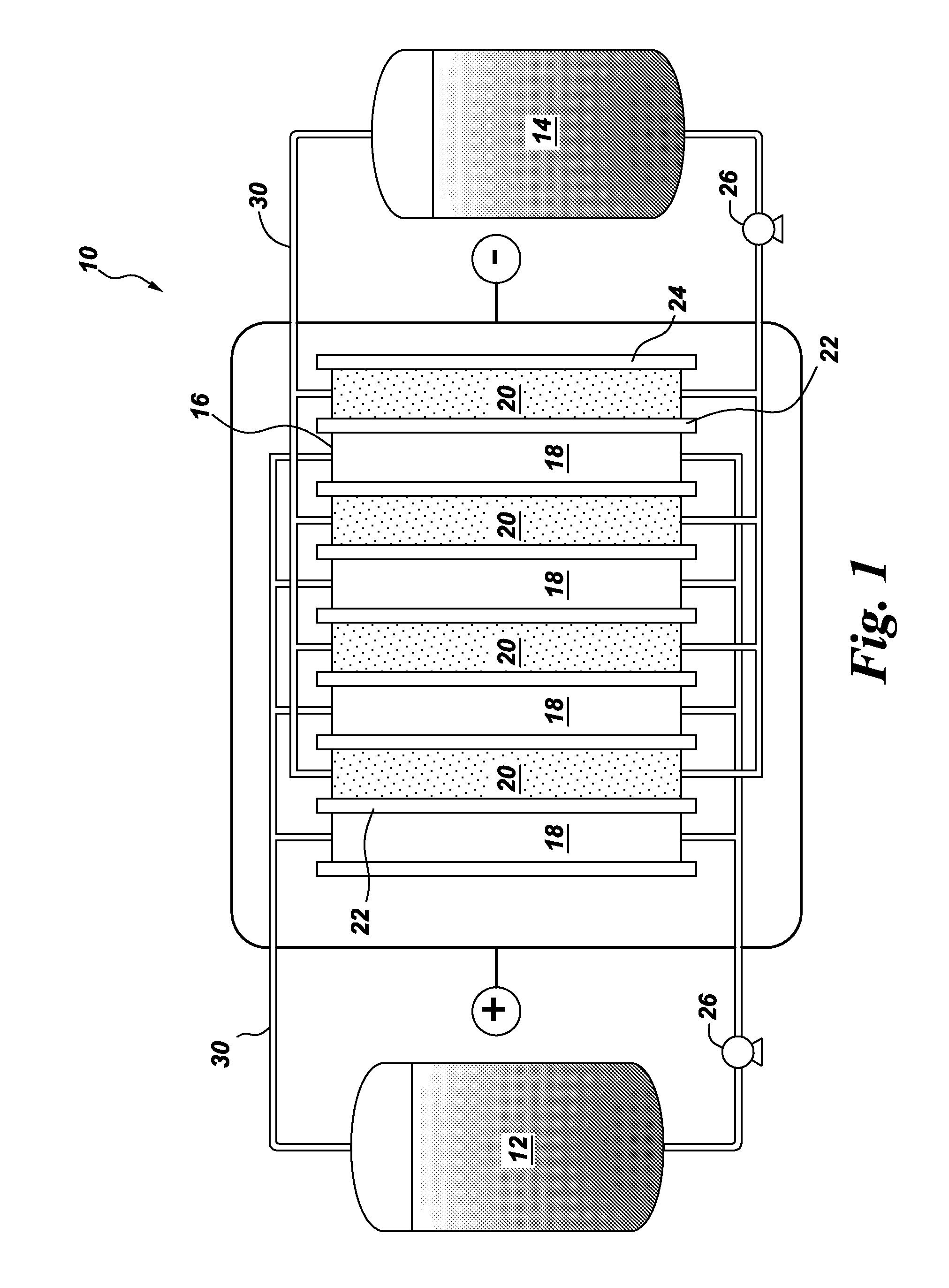
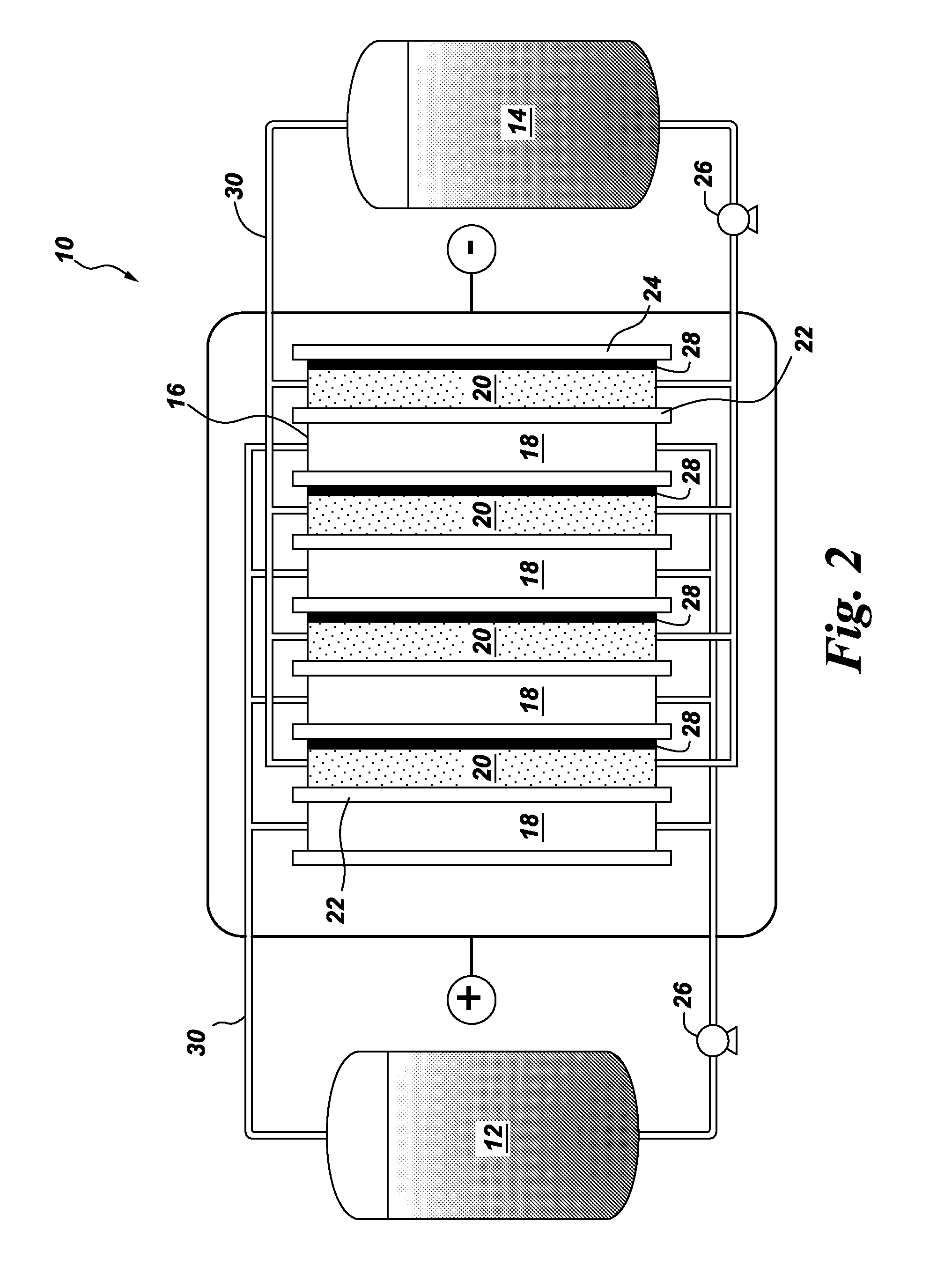
Spiral electrodeionization device with flow distribution profiling
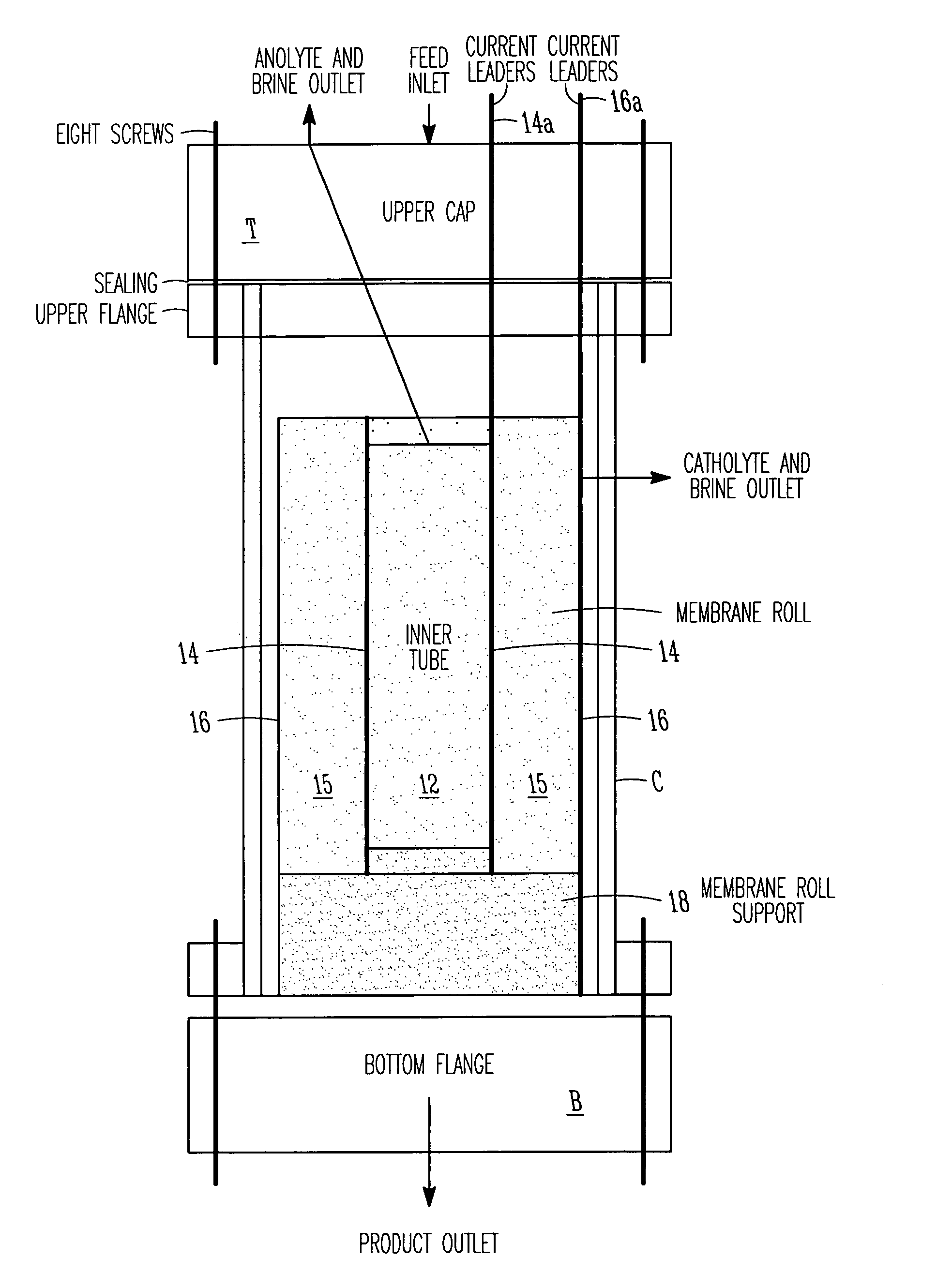
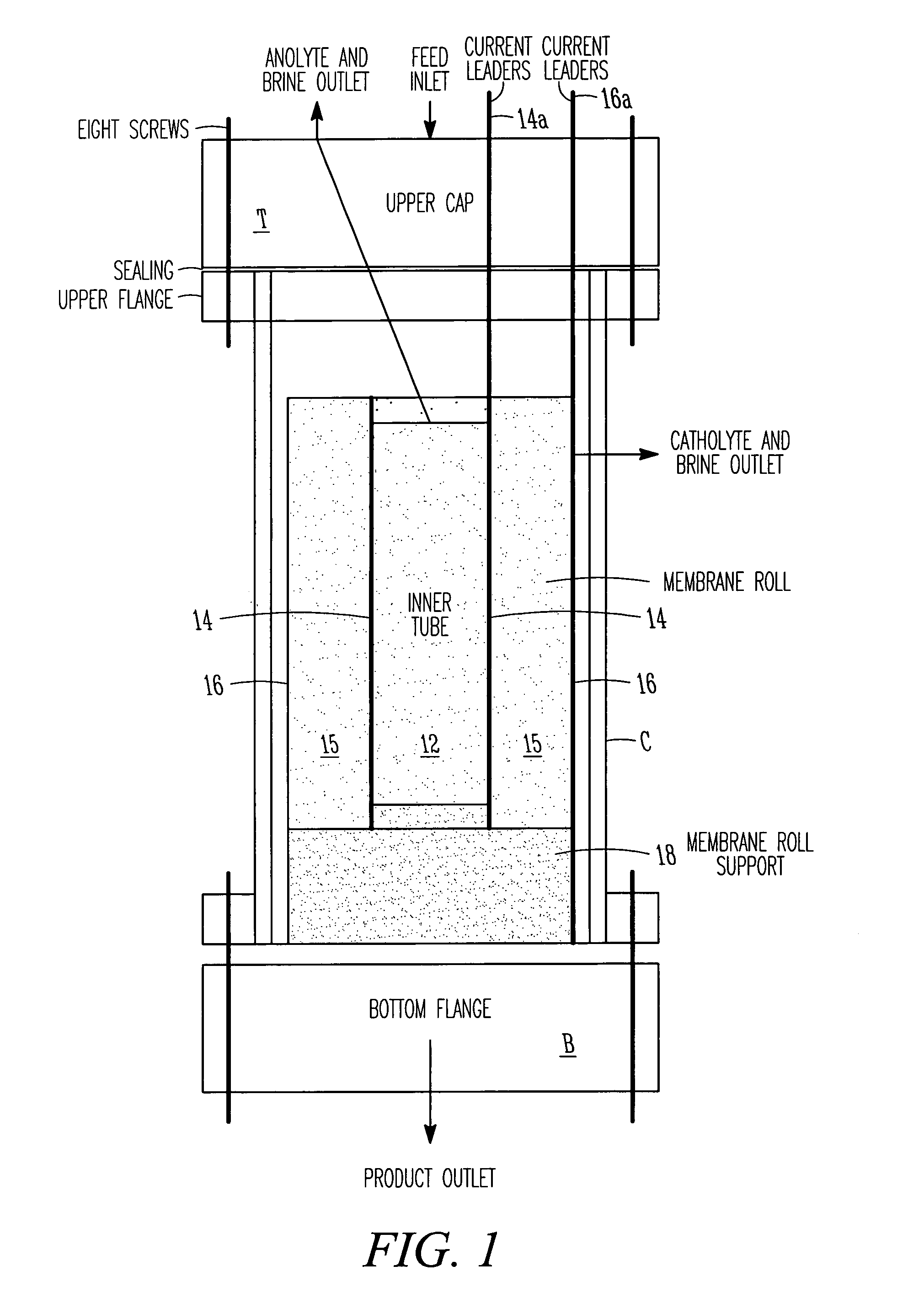
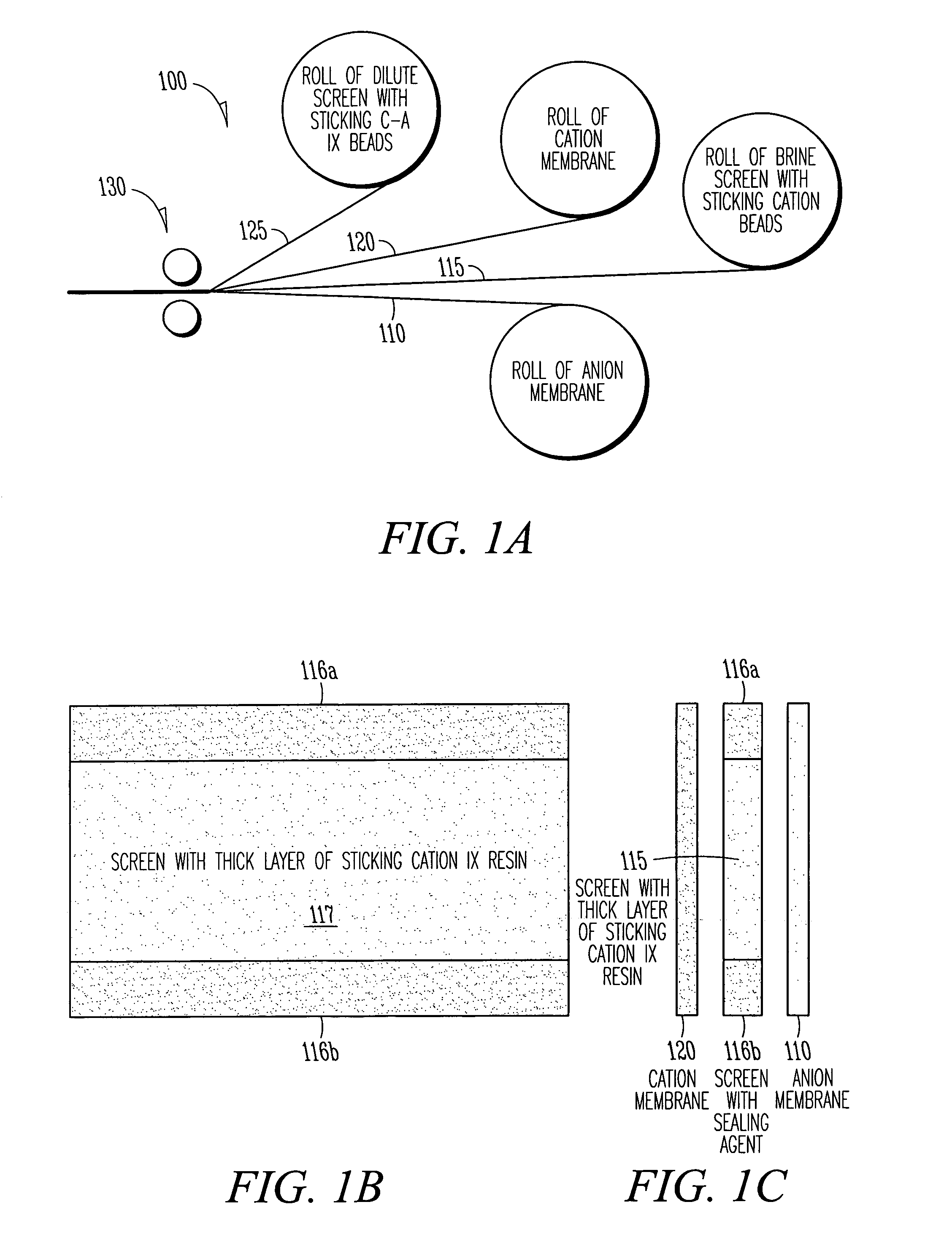
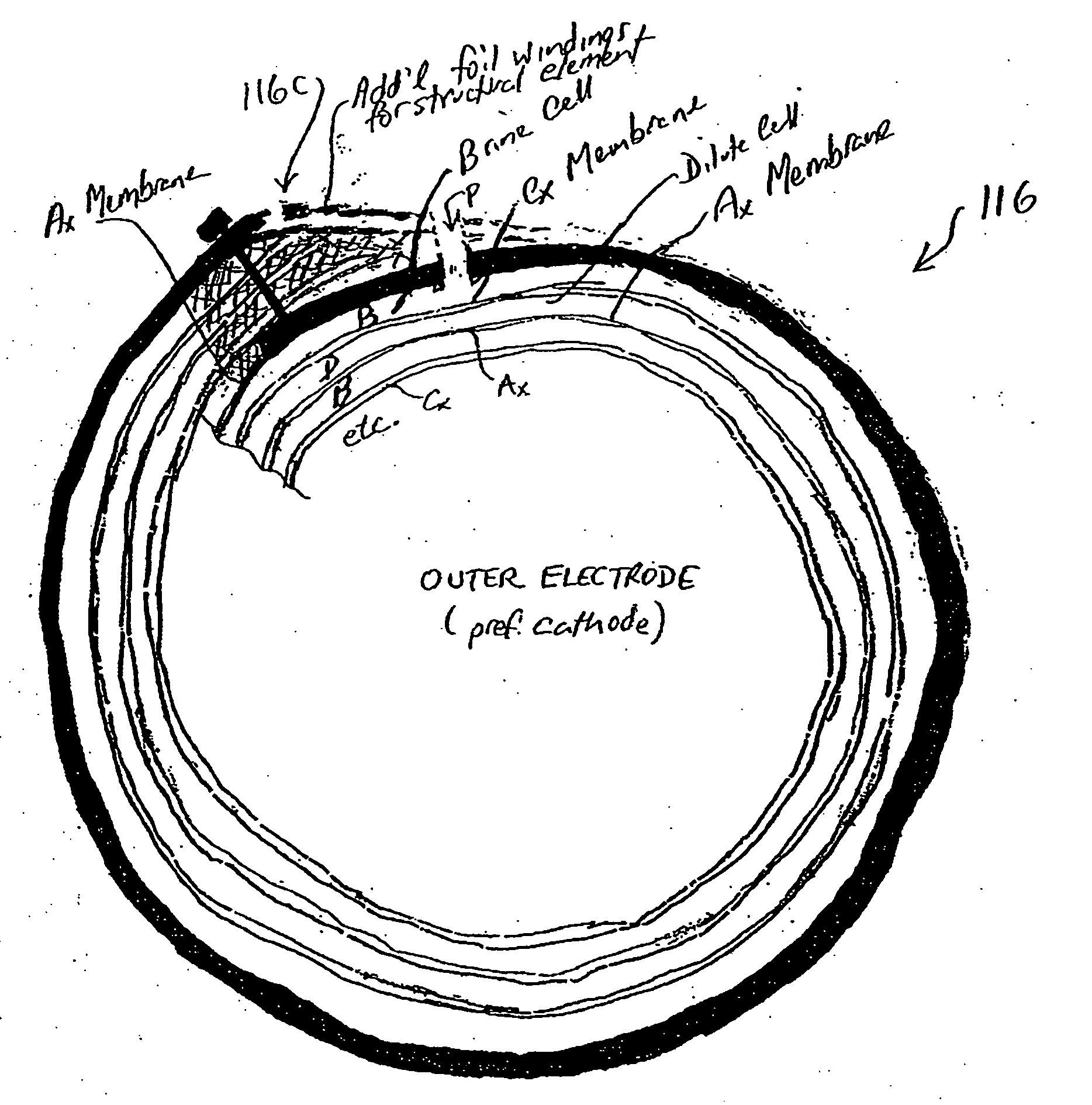
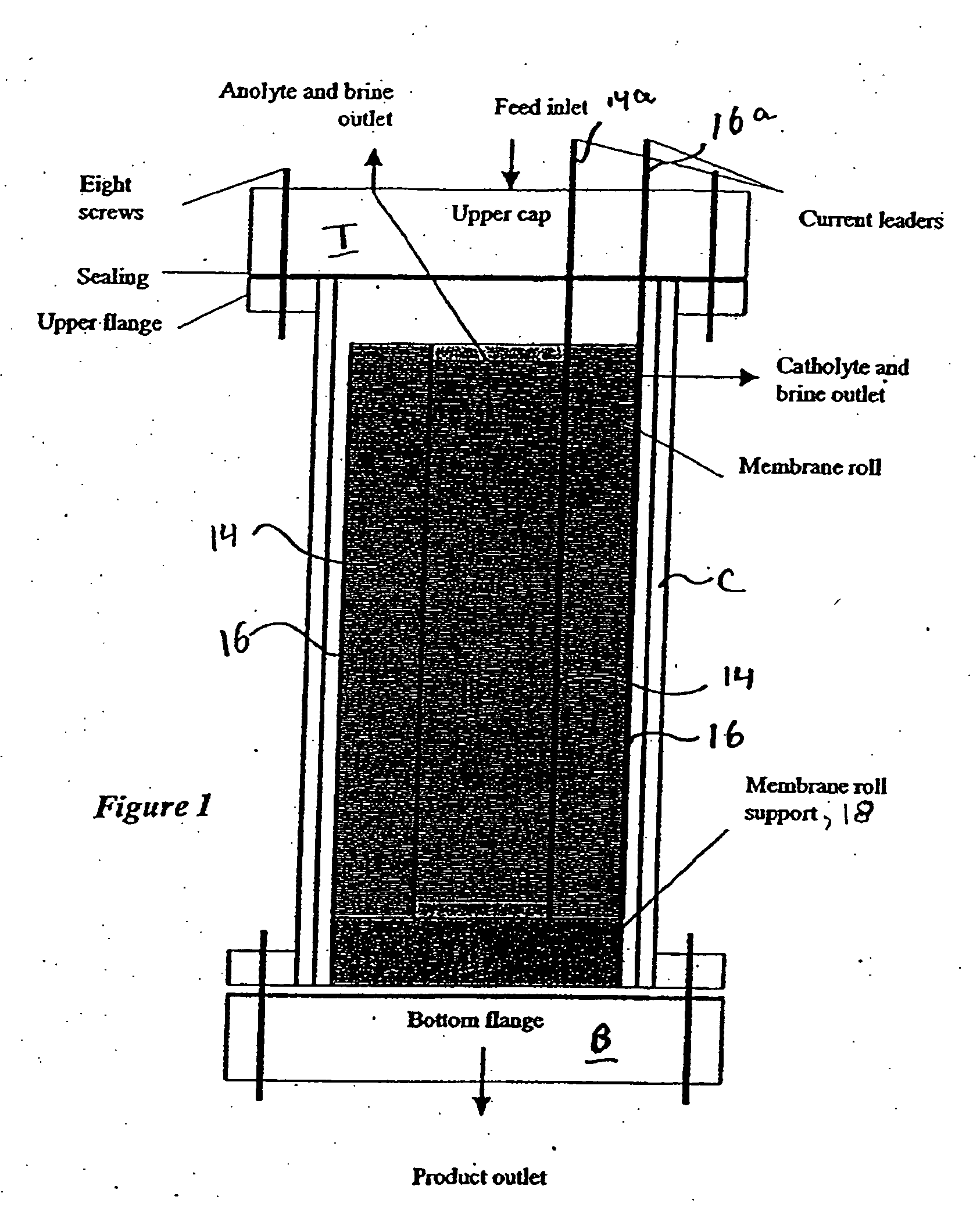
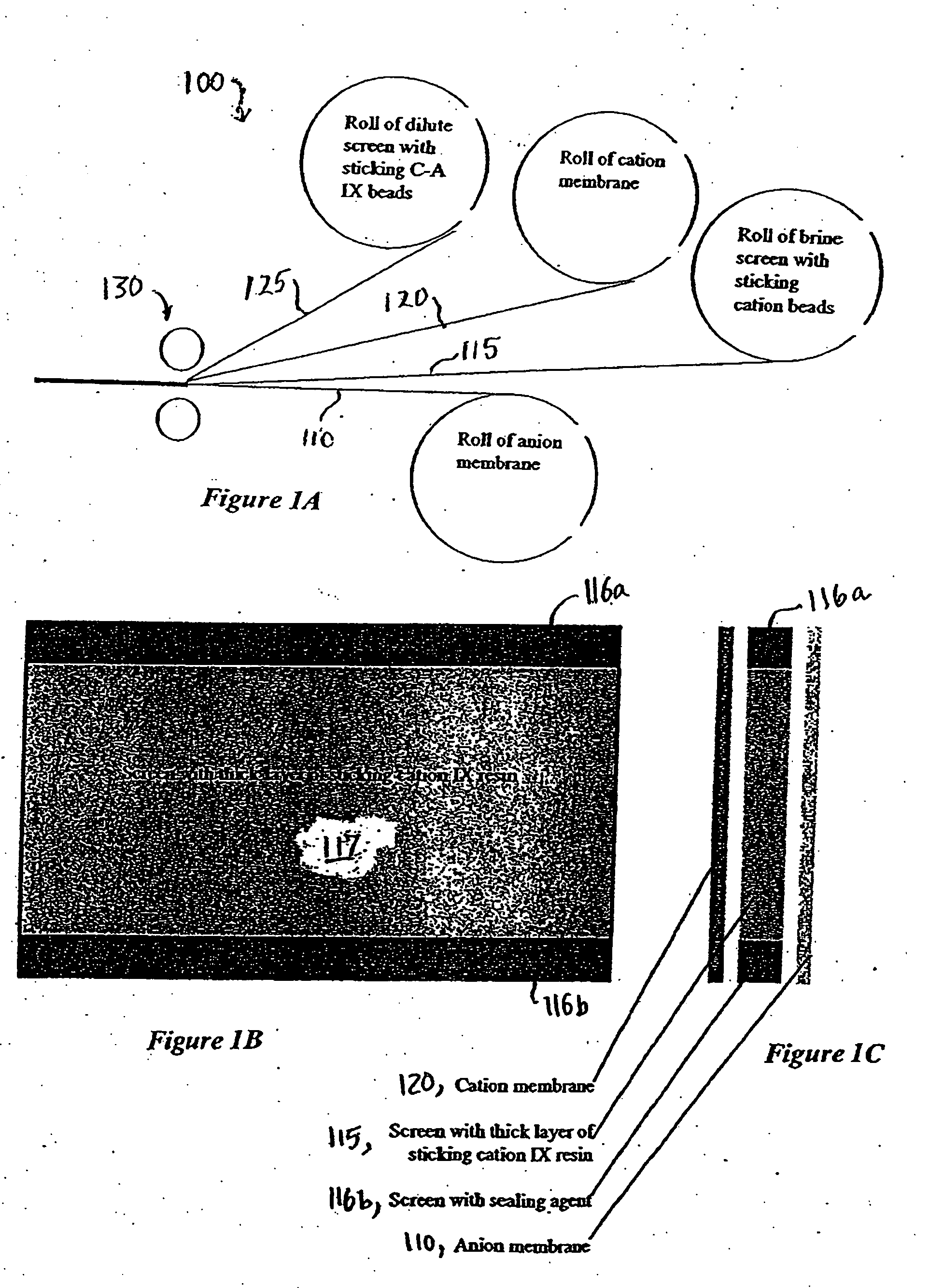
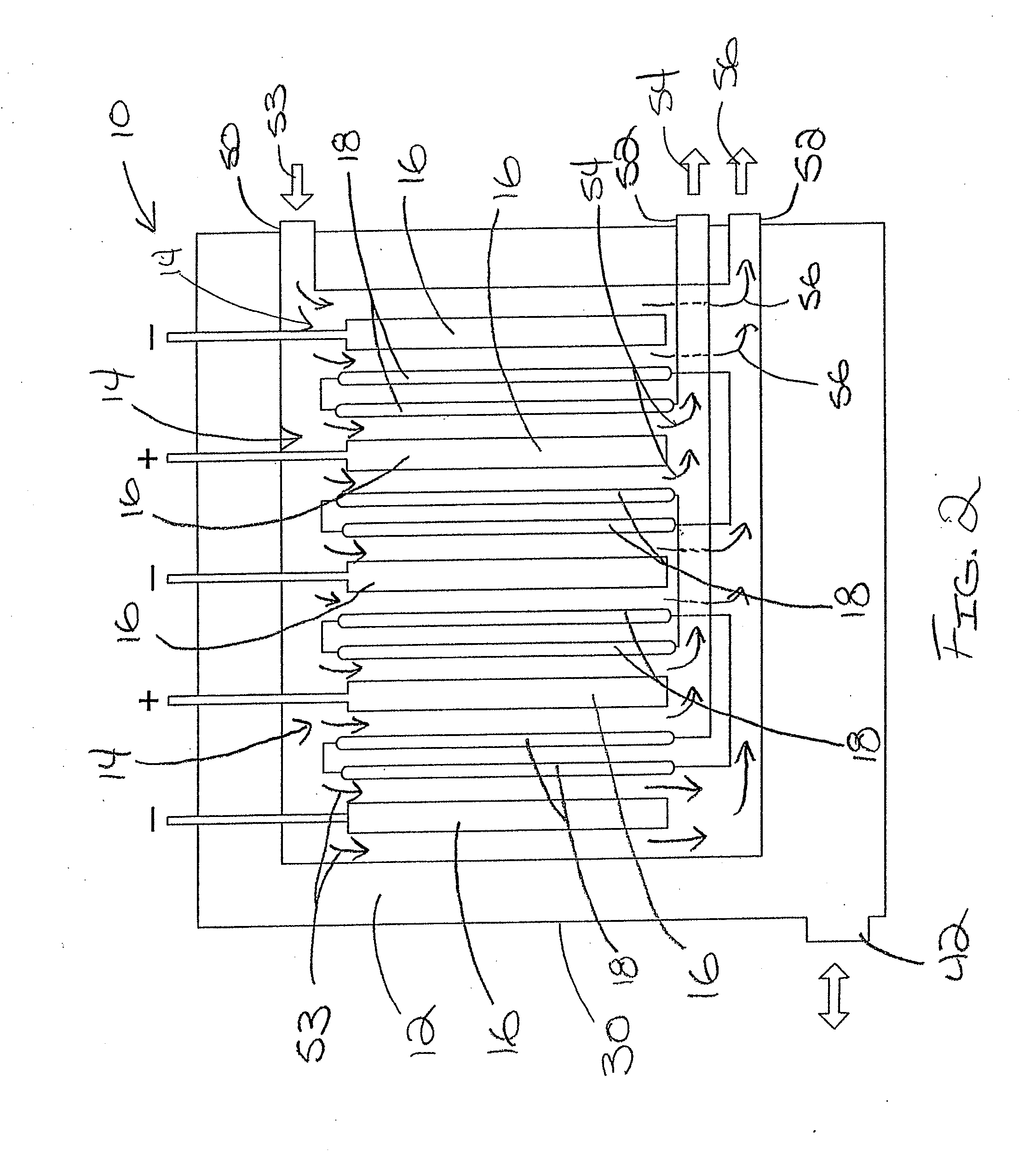
ActiveUS7306709B2Easy to manufactureGood removal effectCellsIon-exchange column/bed processesFiberFlow cell
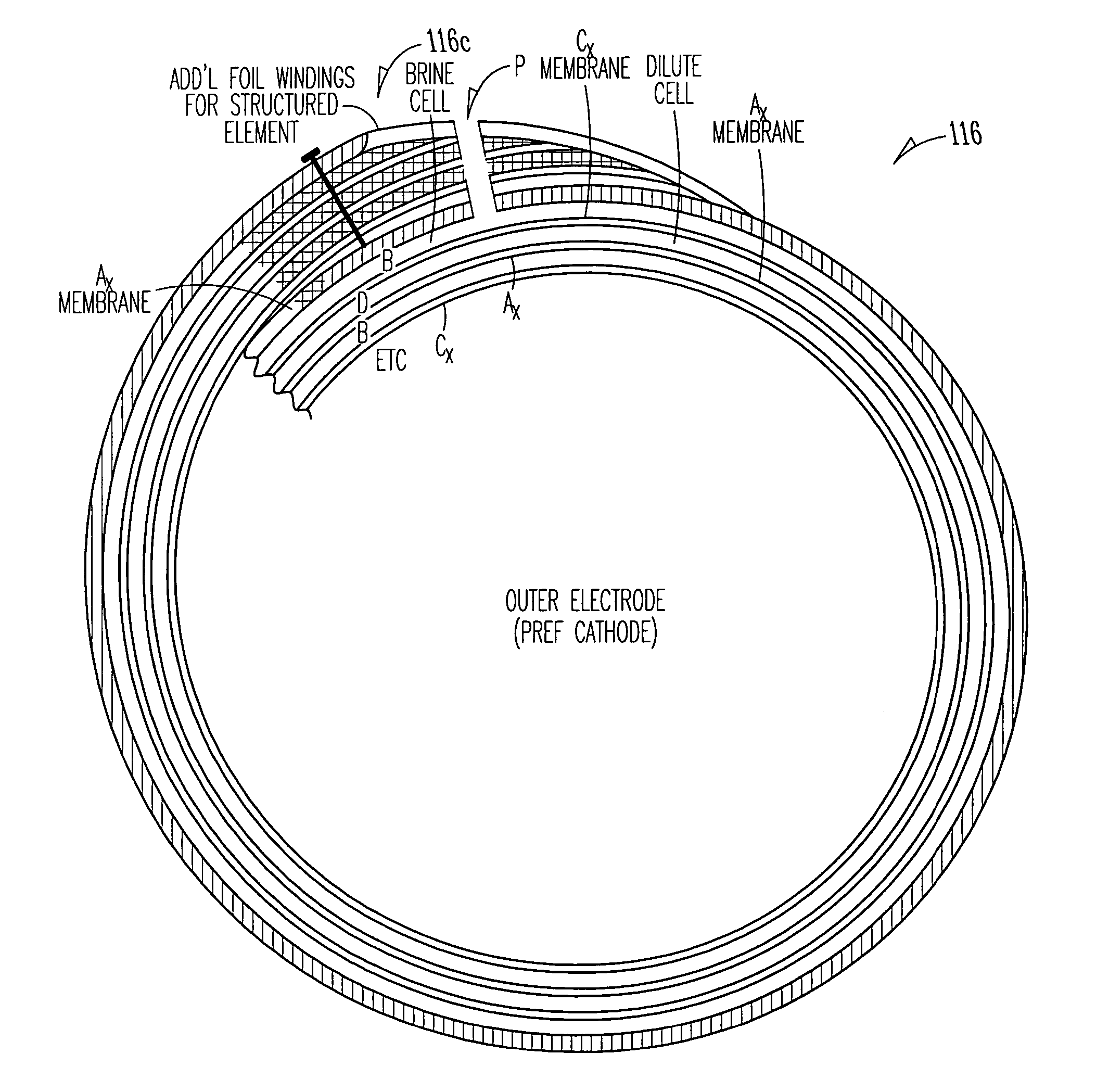
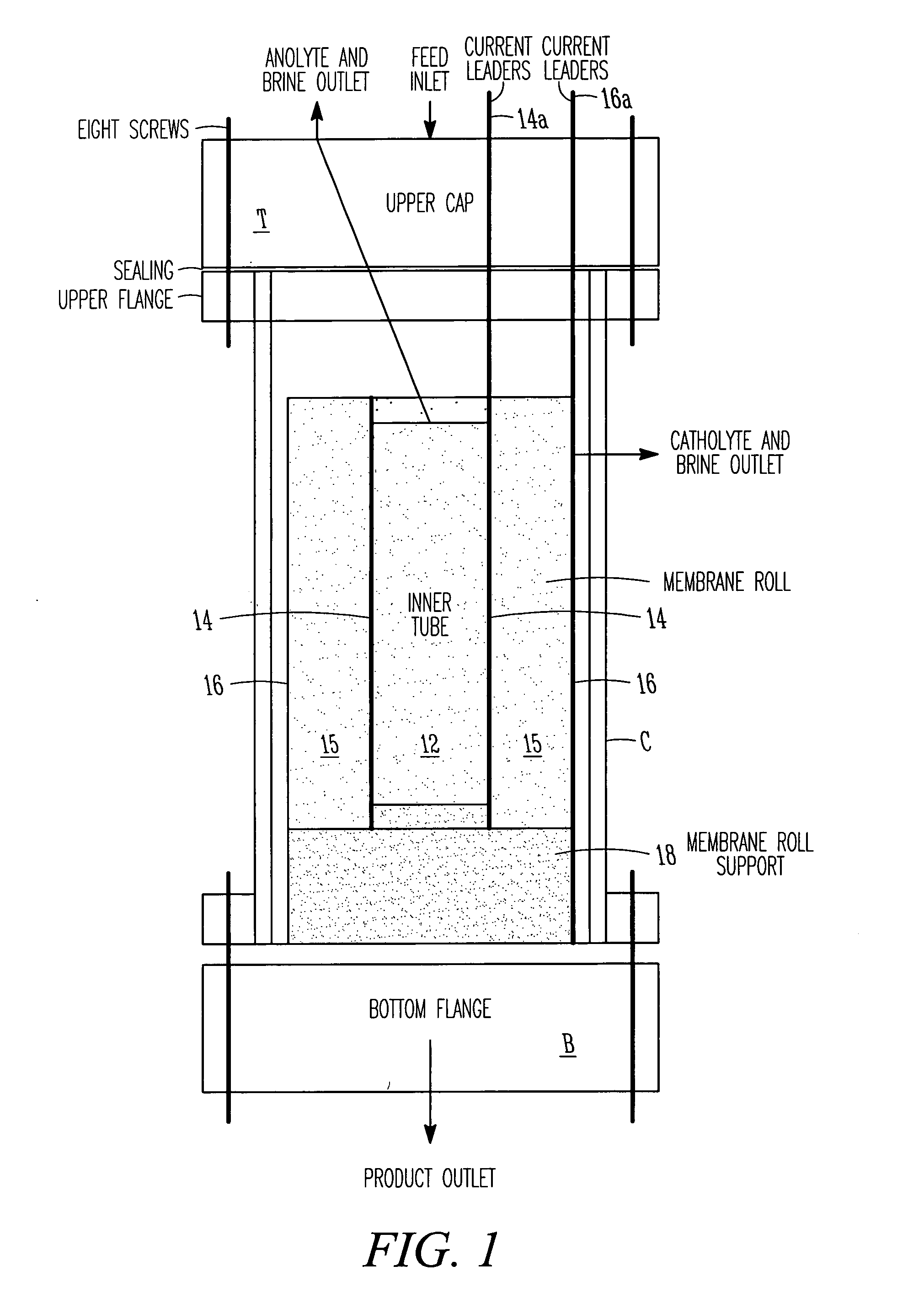
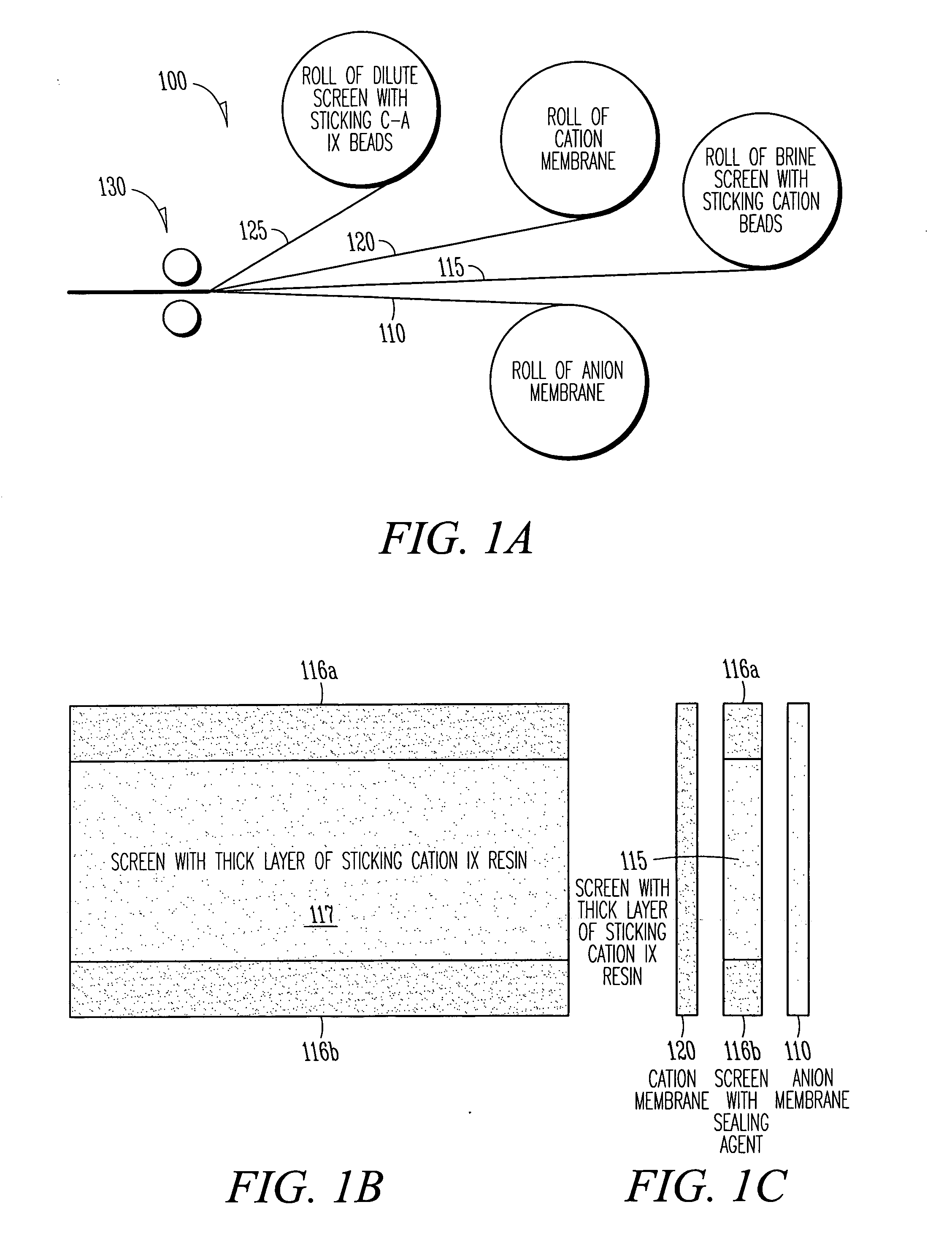
EDI apparatus for demineralizing a liquid flow is assembled in a housing having a cylindrical shape, and includes two metal electrodes, and one or more leafs, each leaf comprising a pair of selectively ion-permeable membranes arranged parallel to each other and spaced apart by spacing elements that allow liquid to flow in the interstitial space between membranes, thus forming an arrangement of dilute and concentrate cells in a desired flow configuration. Spacing elements between membranes, as well as between leaves, can be formed of inert polymer material, ion exchange beads, ion exchange fibers, a combination of two or more these elements, or a porous media incorporating one or more of such elements as an intrinsic part. An inner or central electrode and an outer or perimeter electrode establish a generally uniform and radially-oriented electrical or ionic current between the inner and the outer electrodes, across the helical flow spaces defined by the membrane / spacer windings. One or both electrodes may include a pocket, and the adjacent flow cells lie parallel to the electrode and free of shadowing and field inhomogeneity around a full circumference of the electrode. Flow paths within the helical cells are defined by barrier seals, which may form a path-lengthening maze, while unfilled cell regions may disperse or collect flow within a cell and define pressure gradients promote directional flows. Impermeable barriers between membranes further prevent the feed and concentrate flows from mixing. In various embodiments, seals along or between portions of the flow path may define a multi-stage device, may define separate feed and / or concentrate flows for different stages, and / or may direct the feed and concentrate flows along preferred directions which may be co-current, counter-current or cross-current with respect to each other within the apparatus.
Owner:IONICS INC
Spiral electrodeionization device with flow distribution profiling
EDI apparatus for demineralizing a liquid flow is assembled in a housing having a cylindrical shape, and includes two metal electrodes, and one or more leafs, each leaf comprising a pair of selectively ion-permeable membranes arranged parallel to each other and spaced apart by spacing elements that allow liquid to flow in the interstitial space between membranes, thus forming an arrangement of dilute and concentrate cells in a desired flow configuration. Spacing elements between membranes, as well as between leaves, can be formed of inert polymer material, ion exchange beads, ion exchange fibers, a combination of two or more these elements, or a porous media incorporating one or more of such elements as an intrinsic part. An inner or central electrode and an outer or perimeter electrode establish a generally uniform and radially-oriented electrical or ionic current between the inner and the outer electrodes, across the helical flow spaces defined by the membrane / spacer windings. One or both electrodes may include a pocket, and the adjacent flow cells lie parallel to the electrode and free of shadowing and field inhomogeneity around a full circumference of the electrode. Flow paths within the helical cells are defined by barrier seals, which may form a path-lengthening maze, while unfilled cell regions may disperse or collect flow within a cell and define pressure gradients promote directional flows. Impermeable barriers between membranes further prevent the feed and concentrate flows from mixing. In various embodiments, seals along or between portions of the flow path may define a multi-stage device, may define separate feed and / or concentrate flows for different stages, and / or may direct the feed and concentrate flows along preferred directions which may be co-current, counter-current or cross-current with respect to each other within the apparatus.
Owner:IONICS INC
Microbial fuel cell and membrane cassette for microbial fuel cells
InactiveUS20120003504A1Efficient degradationReduce harmFuel cell auxillariesBiochemical fuel cellsMicrobial fuel cellFuel cells
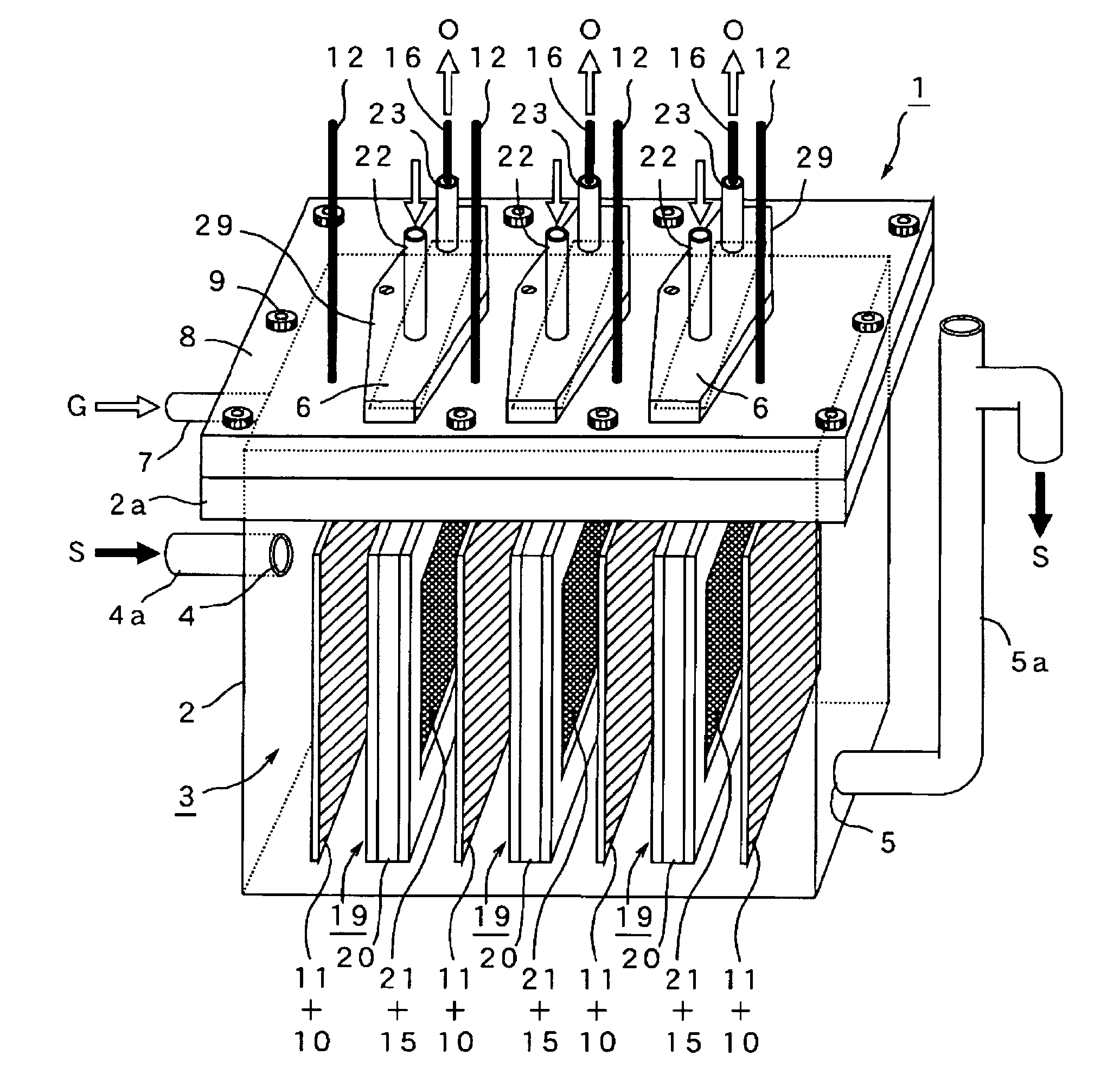
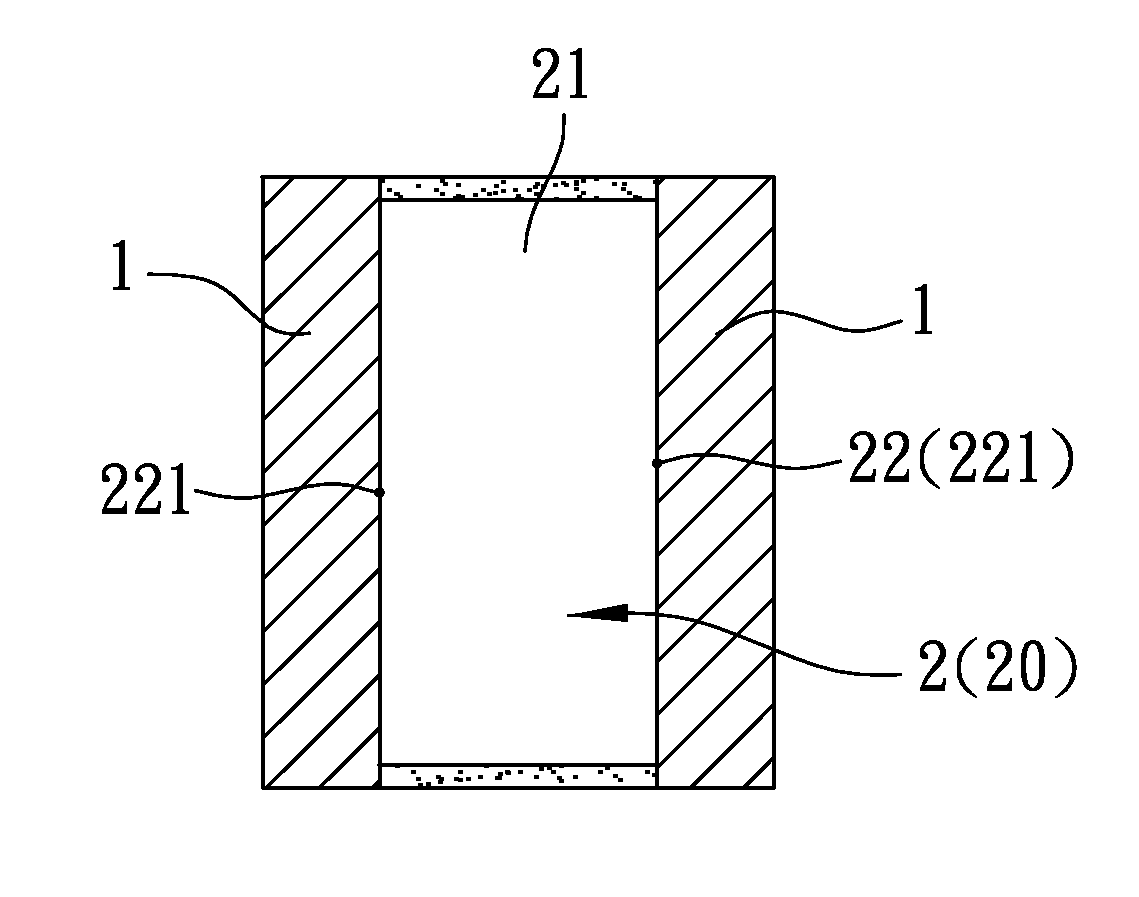
[PROBLEMS] To provide a microbial fuel cell whose parts can be replaced without lowering the energy recovery efficiency and a membrane cassette for microbial fuel cells. [MEANS FOR SOLVING PROBLEMS] A negative electrode (10) supporting anaerobic microorganisms (11) is immersed in an organic substrate (S). A positive electrode (15) sealed together with an electrolyte (D) in a closed hollow cassette (20) having an outer shell (25) at least a part of which is formed of an ion-permeable membrane (21), an inlet (22), and an outlet (23) or connected to the inner side of an ion-permeable membrane (21) is inserted into the organic substrate (S). While oxygen (O) is supplied into the cassette (20) through the inlet (22) and the outlet (23), electricity is taken out through a circuit (18) electrically interconnecting the negative and positive electrodes (10, 15). Preferably, the outer shell (25) of the closed hollow cassette (20) is a hollow outer shell frame (25) having an opening (26) which is closed by stretching an ion-permeable membrane (21), an inlet (22), and an outlet (23), and the ion-permeable membrane (21) is a membrane / electrode assembly (MEA) formed integrally with the positive electrode (15).
Owner:KAJIMA CORP
Process for preparing a solid state electrolyte used in an electrochemical capacitor
InactiveUS20130149436A1Increase capacitanceCapacitor electrolytes/absorbentsSpecial surfacesSolid state electrolytePolymer science
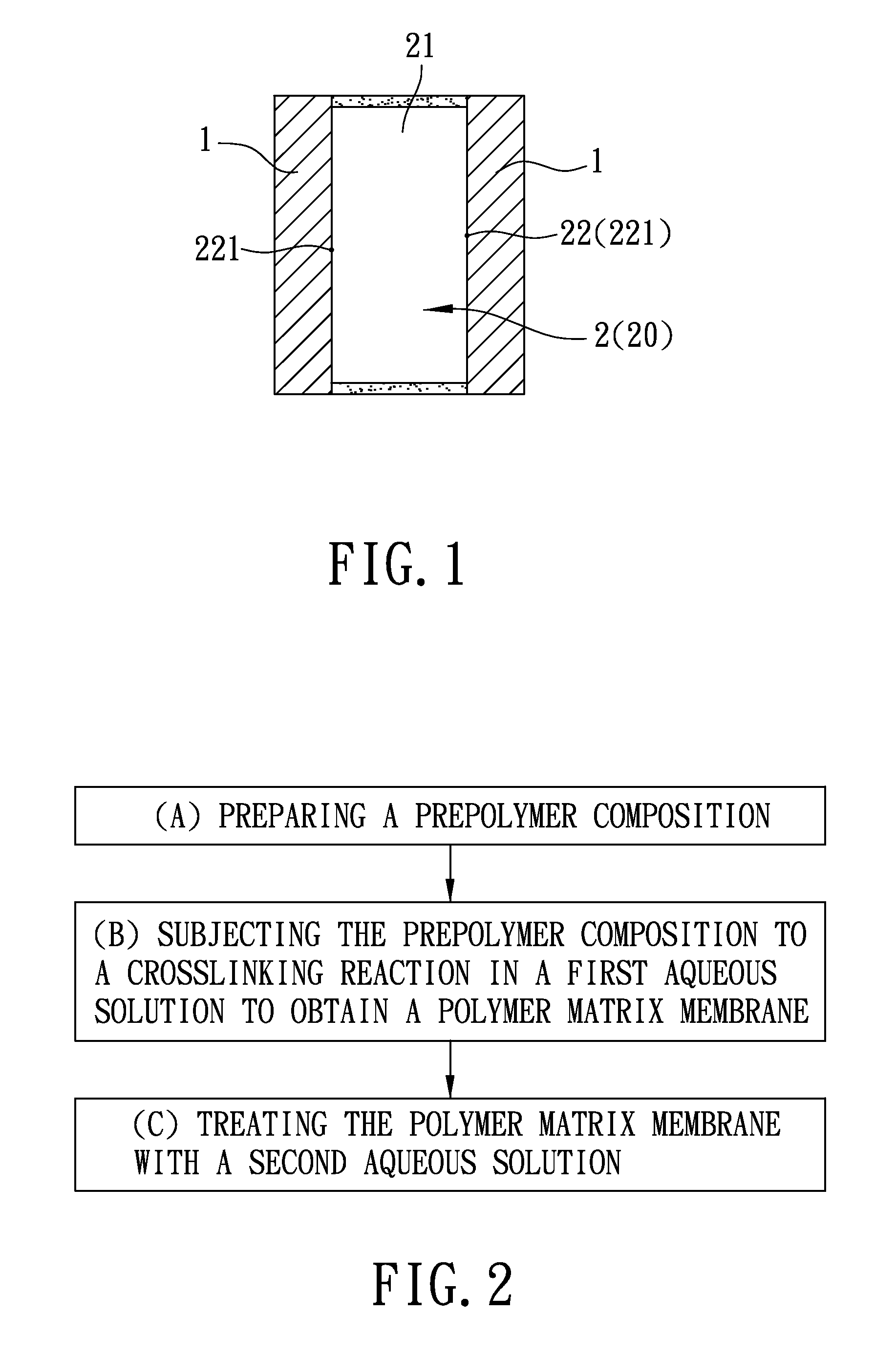
A process for preparing a solid state electrolyte used in an electrochemical capacitor includes the steps of: (a) preparing a prepolymer composition which includes a water-retaining polymer component and a film-forming hydroxyl-containing polymer component; (b) subjecting the prepolymer composition to a crosslinking reaction so as to form a polymer matrix membrane including a polymer matrix and an ion-permeable film; and (c) treating the polymer matrix membrane with an aqueous solution which includes a plurality of positive and negative ions so as to permit the positive and negative ions to permeate the ion-permeable film to be retained in the polymer matrix, thereby forming the solid state electrolyte.
Owner:NAT KAOHSIUNG UNIV OF SCI & TECH
Method for electric field to absorb and purify liquid crystal
InactiveCN101760204AHigh resistivityReduce trace ion contentLiquid crystal compositionsLiquid separation by electricityElectricitySorbent
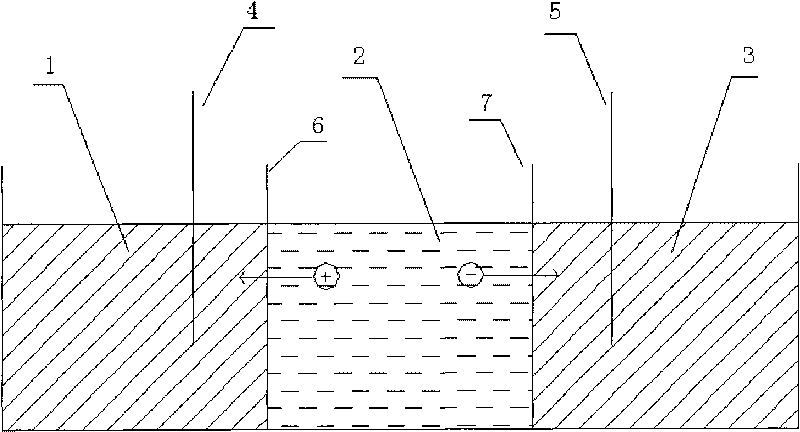
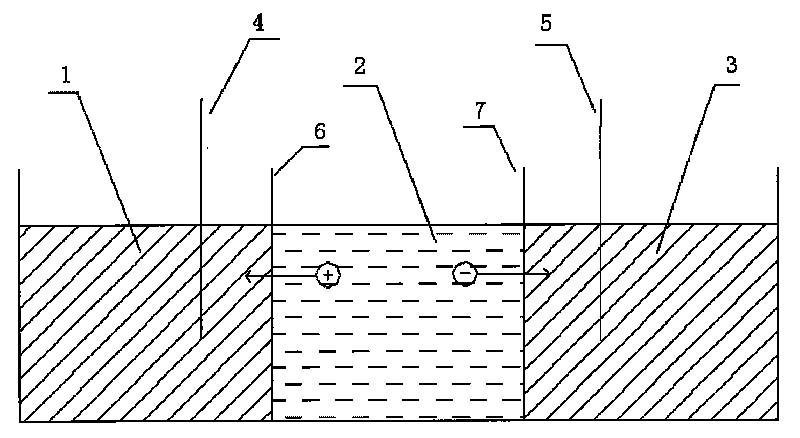
The invention particularly relates to a method for an electric field to absorb and purify a liquid crystal. The method adopts an electric field absorbing and purifying device which is provided with the following three intervals: a cathode solvent chamber, an anode solvent chamber and a middle purifying chamber. Ion permeable membranes are respectively arranged between the cathode solvent chamber and the middle purifying chamber, and between the anode solvent chamber and the middle purifying chamber. A negative electrode is inserted in the cathode solvent chamber, and a positive electrode is inserted in the anode solvent chamber. When the device is applied for purification, liquid crystal material solution is arranged in the middle purifying chamber, absorbing agents and solvents are respectively arranged in the cathode solvent chamber and the anode solvent chamber, DC or AC electricity is switched on between the two electrodes, and finally high-purity liquid crystal materials are extracted. The invention combines the applied electric field method with the absorption method and carries out centralized as well as combined absorption under the function of the applied electric field by respectively utilizing the characteristics and advantages of the two methods, thereby solving the problems that high-resistivity liquid crystal materials are difficult for purification and cannot be continuously as well as stably operated.
Owner:WUHAN POLYTECHNIC UNIVERSITY
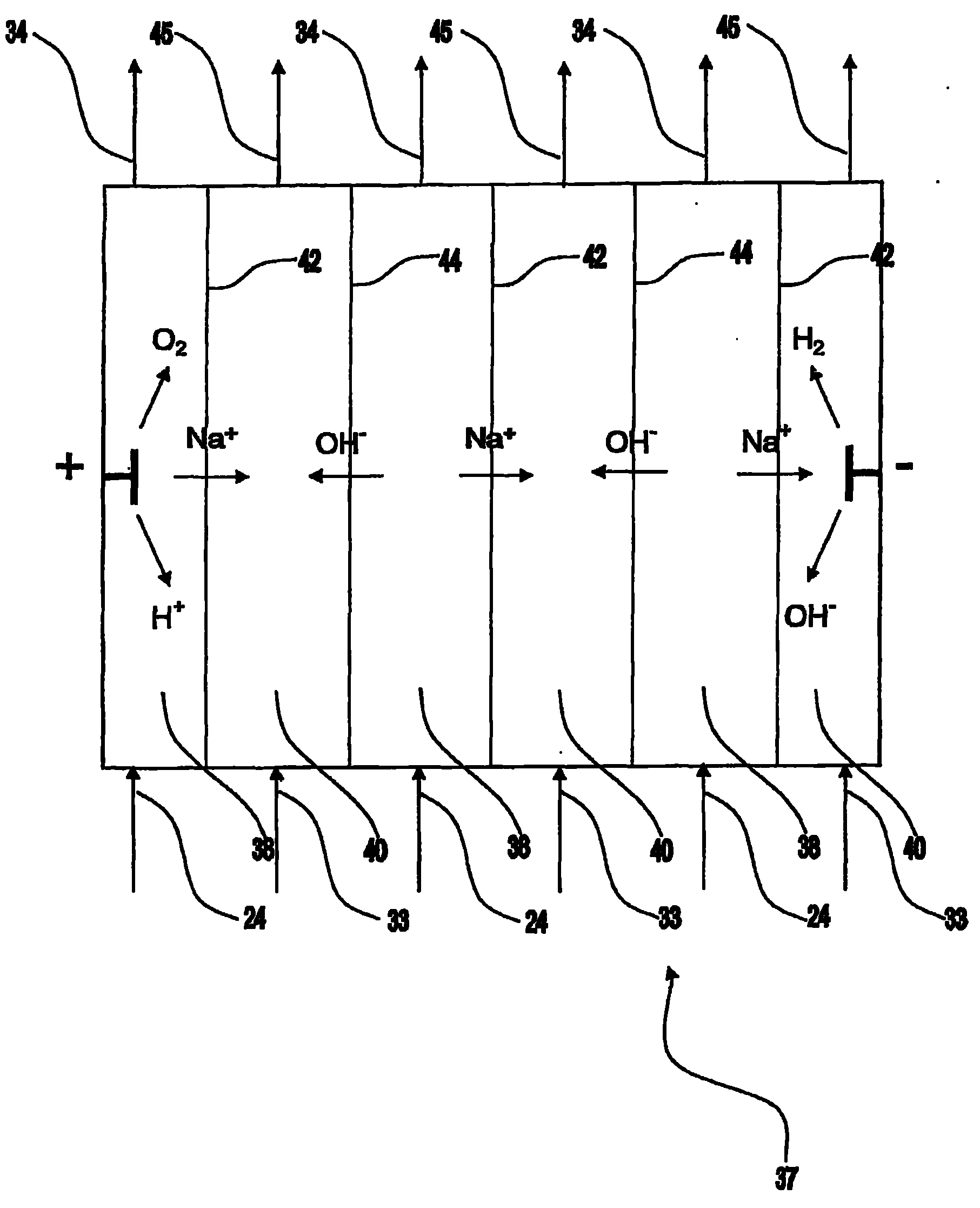
High purity electrodeionization
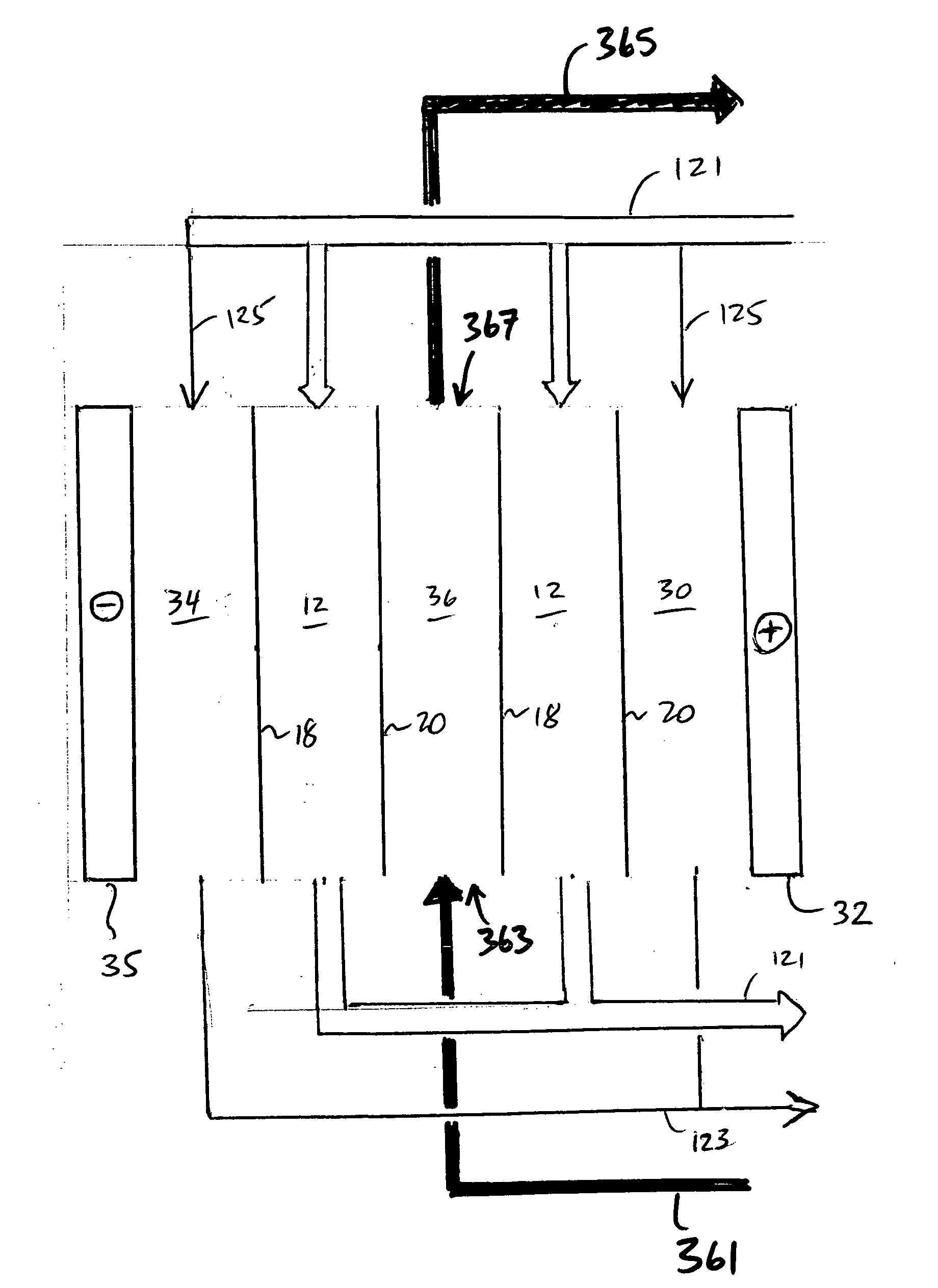
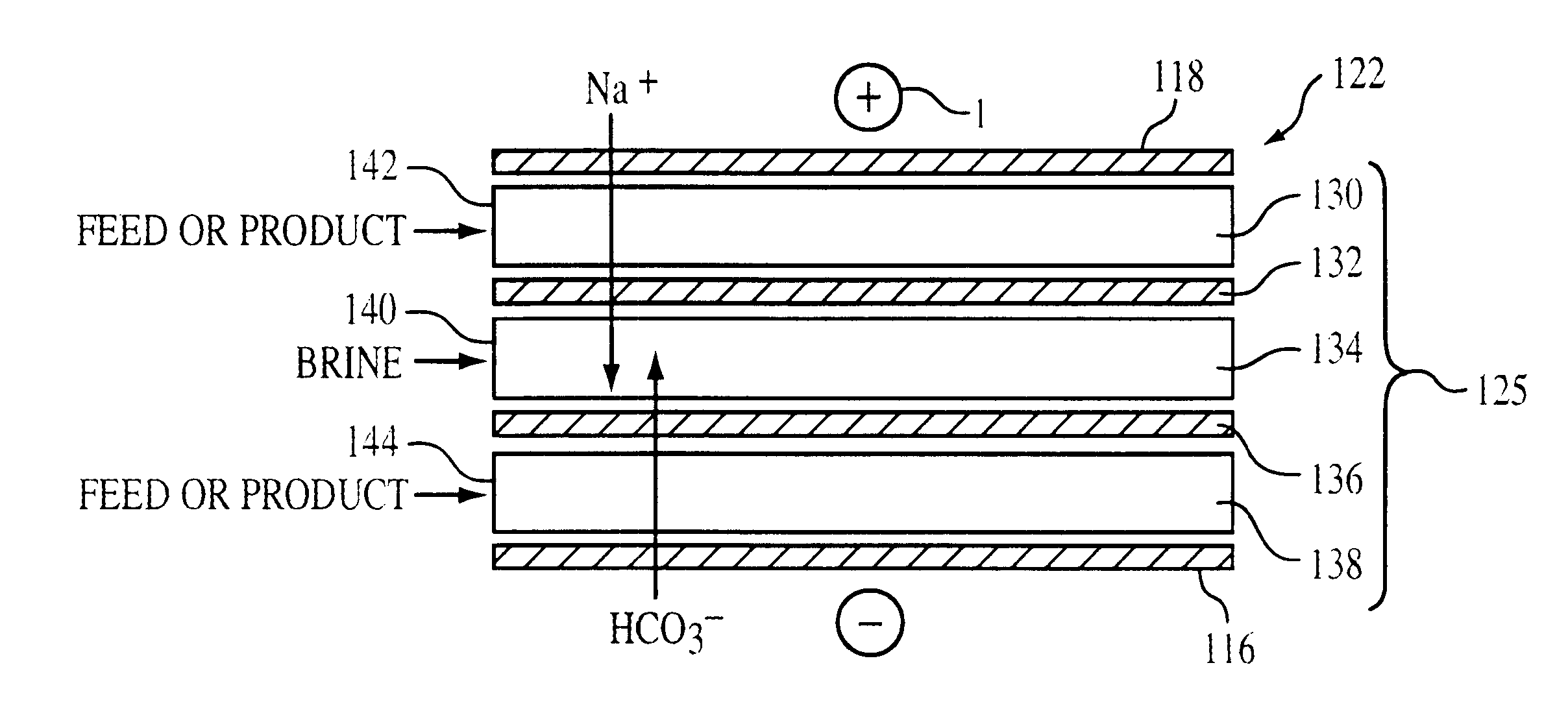
Electrodeionization apparatus for purifying water includes a cathode, an anode, and a plurality of alternating anion permeable membranes and cation permeable membranes between the cathode and anode that define concentrating and diluting flow channels between adjacent pairs of membranes. Each concentrating flow channel includes a first guard channel adjacent to the anion permeable membrane, a second guard channel adjacent to the cation permeable membrane, and a brine channel between the first and second guard channels. The first and second guard channels have water with lower concentration of dissolved ions than water in the brine channel so as limit parasitic transfer from a concentrating flow channel to a diluting flow channel.
Owner:IONICS INC
Microbial fuel cell treatment of ethanol fermentation process water
ActiveUS8192854B2Economical, convenient, and environmentally friendly methodIncrease productionBacteriaBiofuelsMicrobial fuel cellFuel cells
Owner:UT BATTELLE LLC
Spiral electrodeionization device with segregated ionic flows
ActiveUS20060169580A1Easy to manufactureGood removal effectCellsIon-exchange column/bed processesFiberPorous medium
Owner:IONICS INC
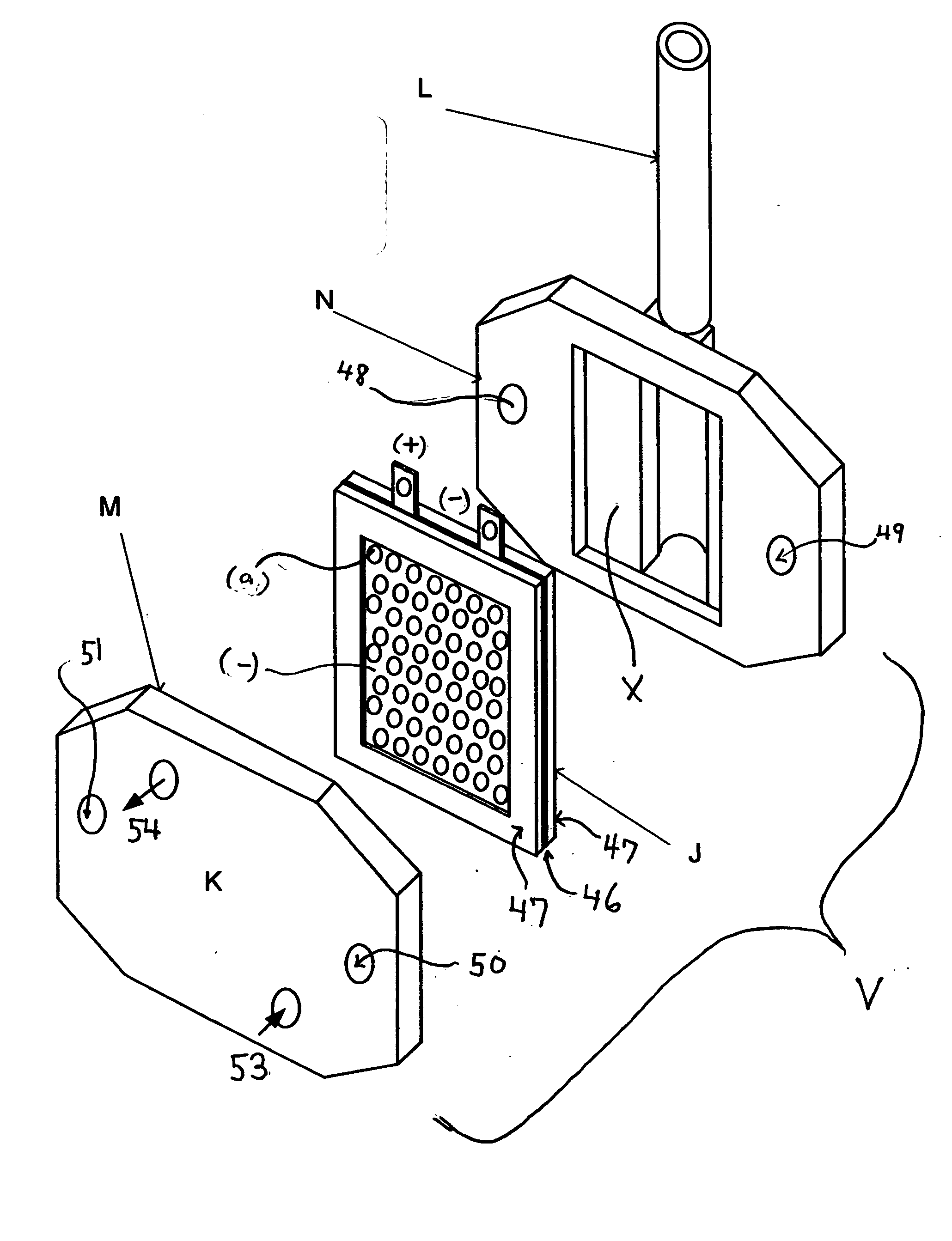
Electrolyzed water generator and electrode set with membrane used in the electrolyzed water generator
InactiveCN101437762AStable characteristicsCellsWater/sewage treatment by electrochemical methodsElectrolysisEngineering
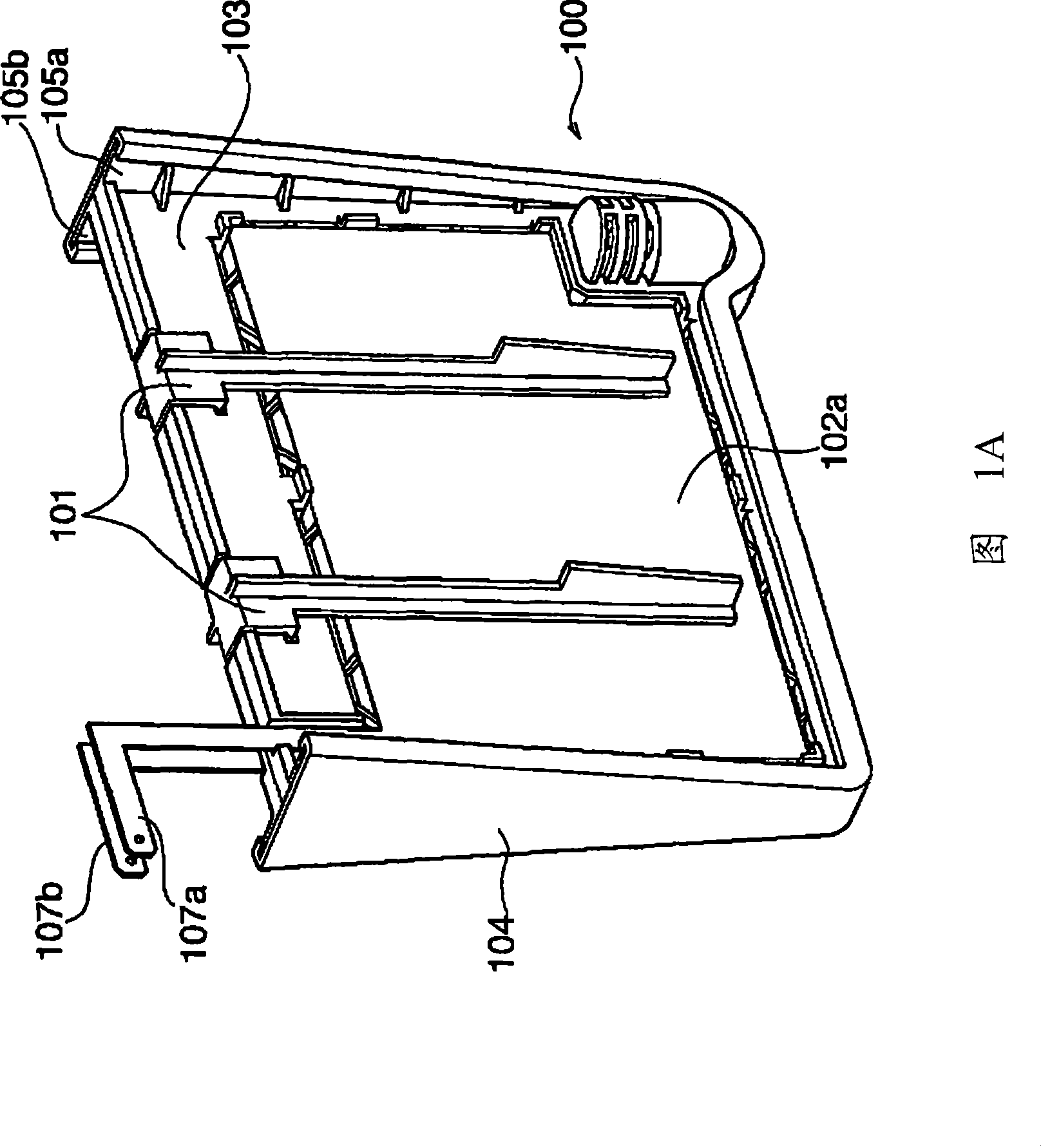
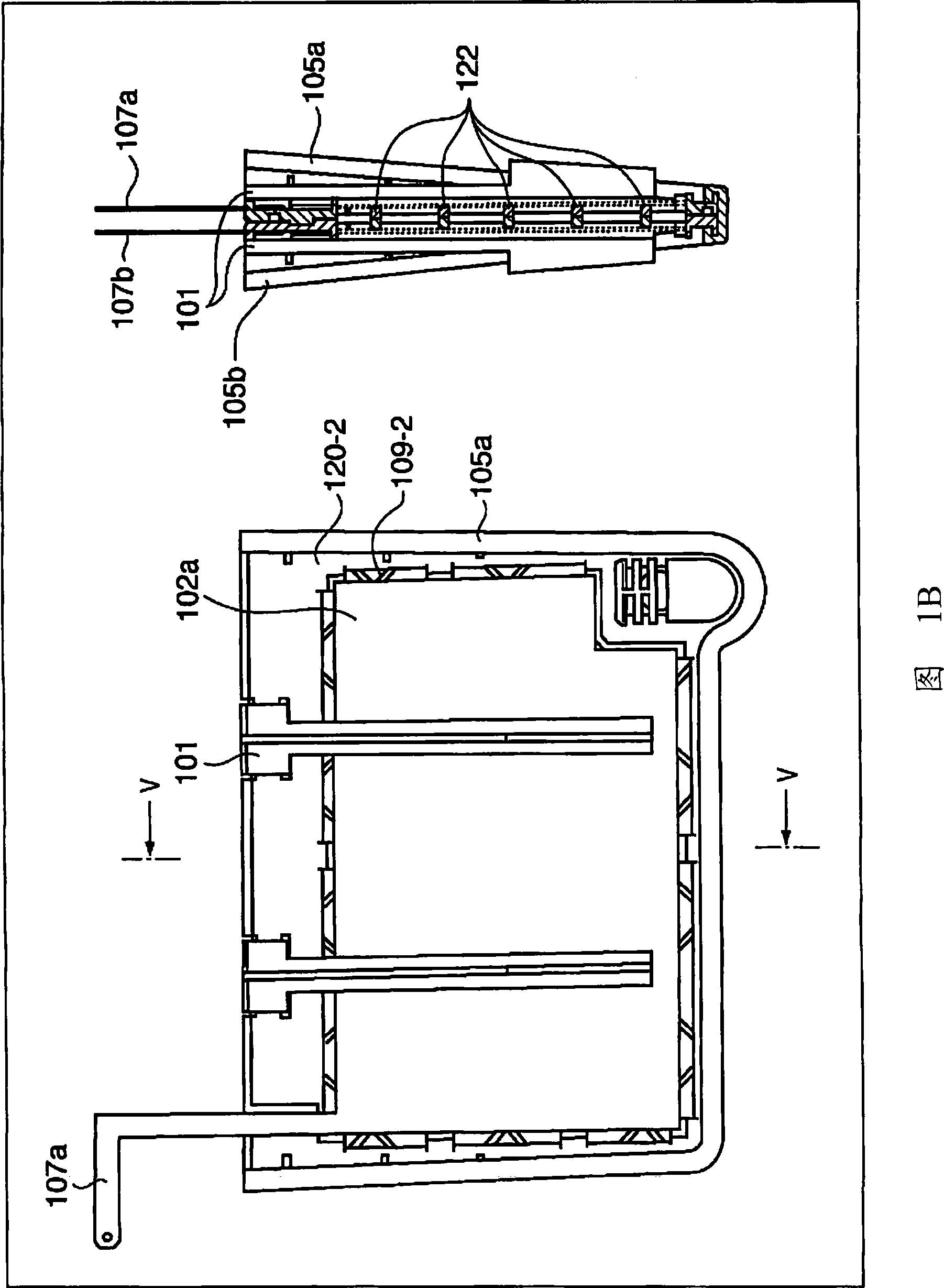
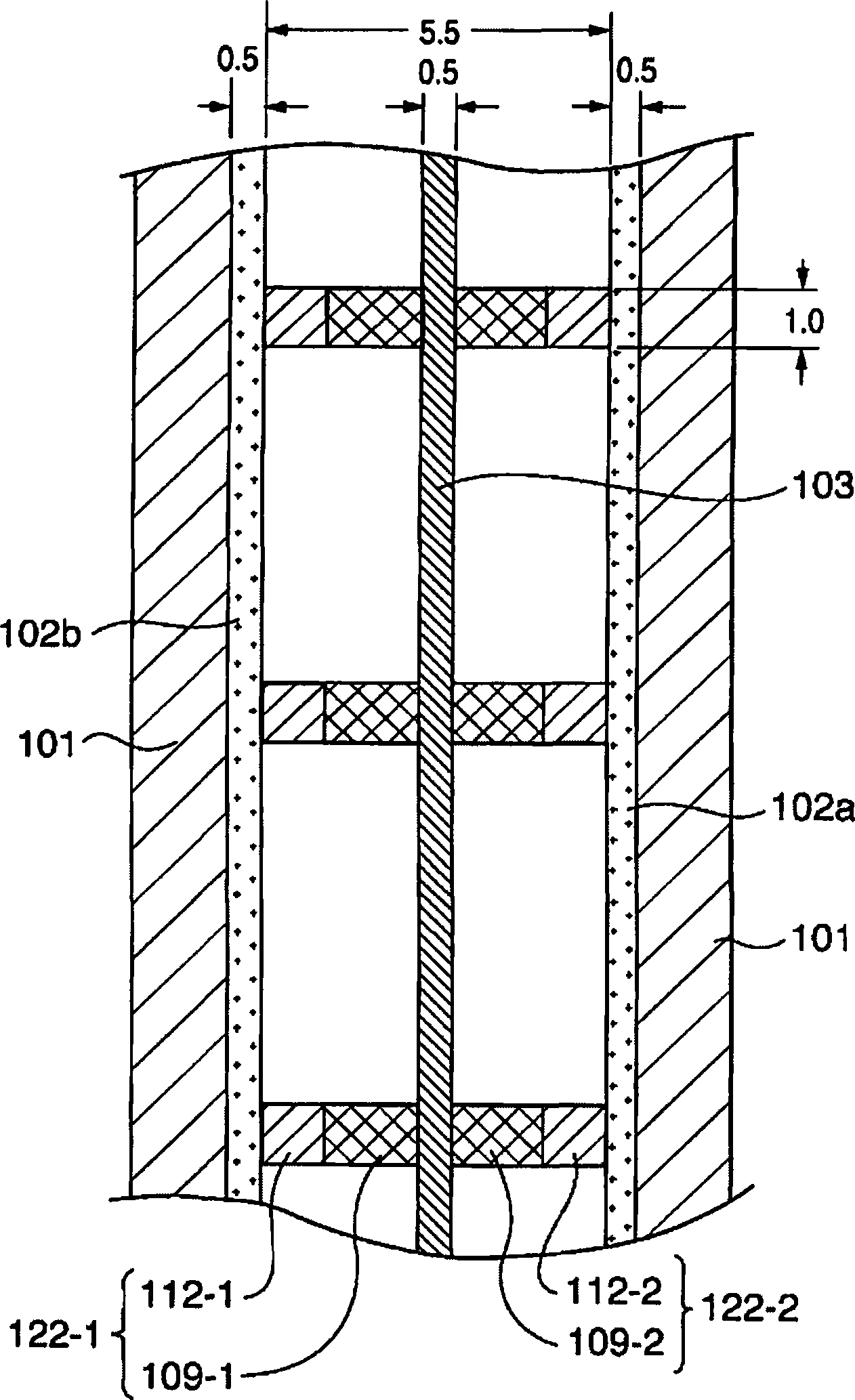
There is provided an electrolyzed water generator capable of performing electrolysis in a stable manner for a long period of time. The electrode set (100) with a membrane in the electrolyzed water generator has membrane supporting plates (105a, 105b) by which an ion permeable membrane (103) is secured and two electrodes (102a, 102b) disposed on both sides of the membrane (103). The two electrodes (102a, 102b) are pressed and secured by a clip to a spacing portion disposed at the openings of the membrane supporting plates (105a, 105b). As a result, since the distance between the two electrodes (102a, 102b) is kept constant, even if electrolyzed water is repeatedly generated a hundred times, the degradation of the characteristics of the electrolyzed water is not recognized.
Owner:COCOROCA
Electrolyzing system
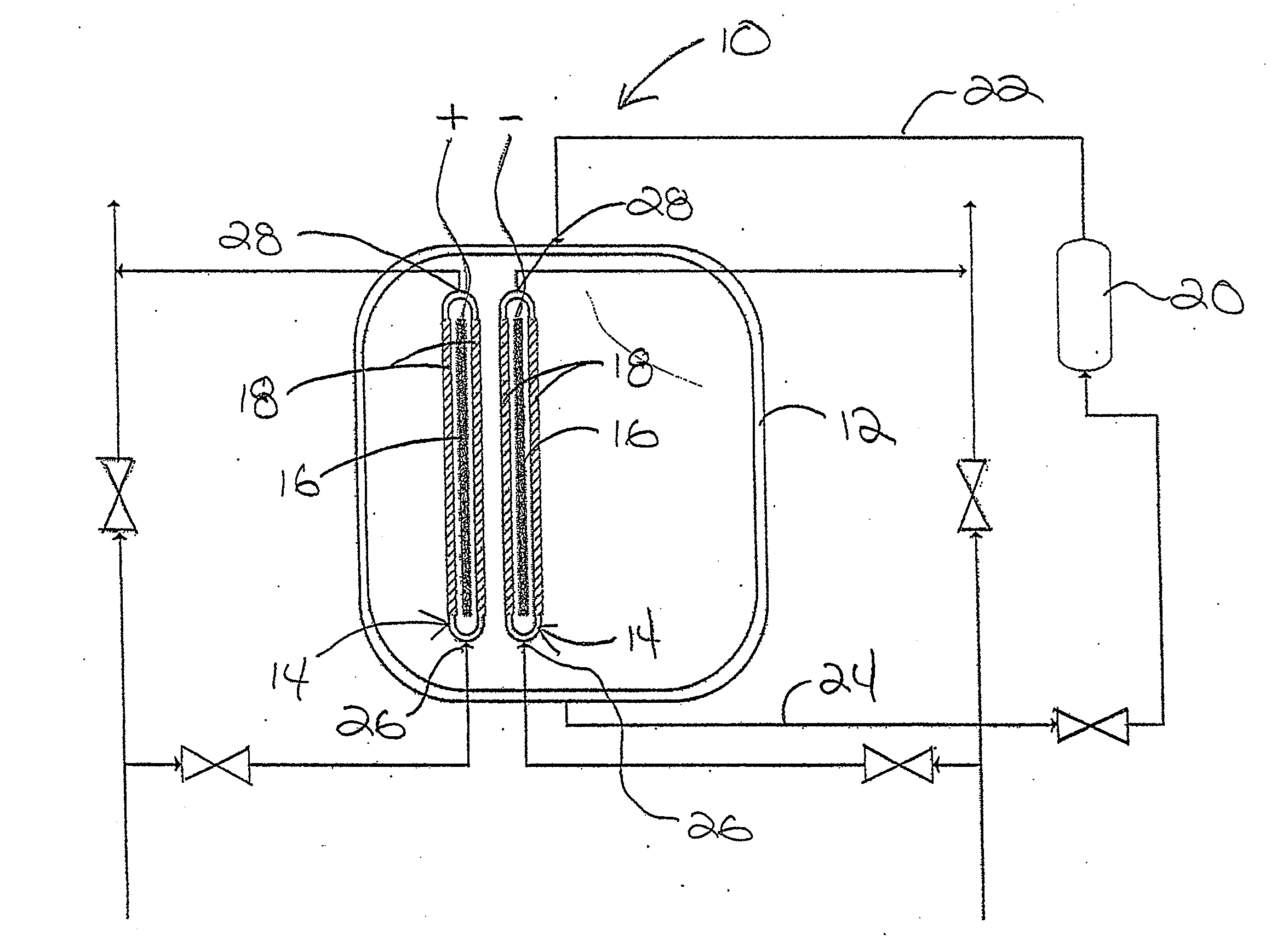
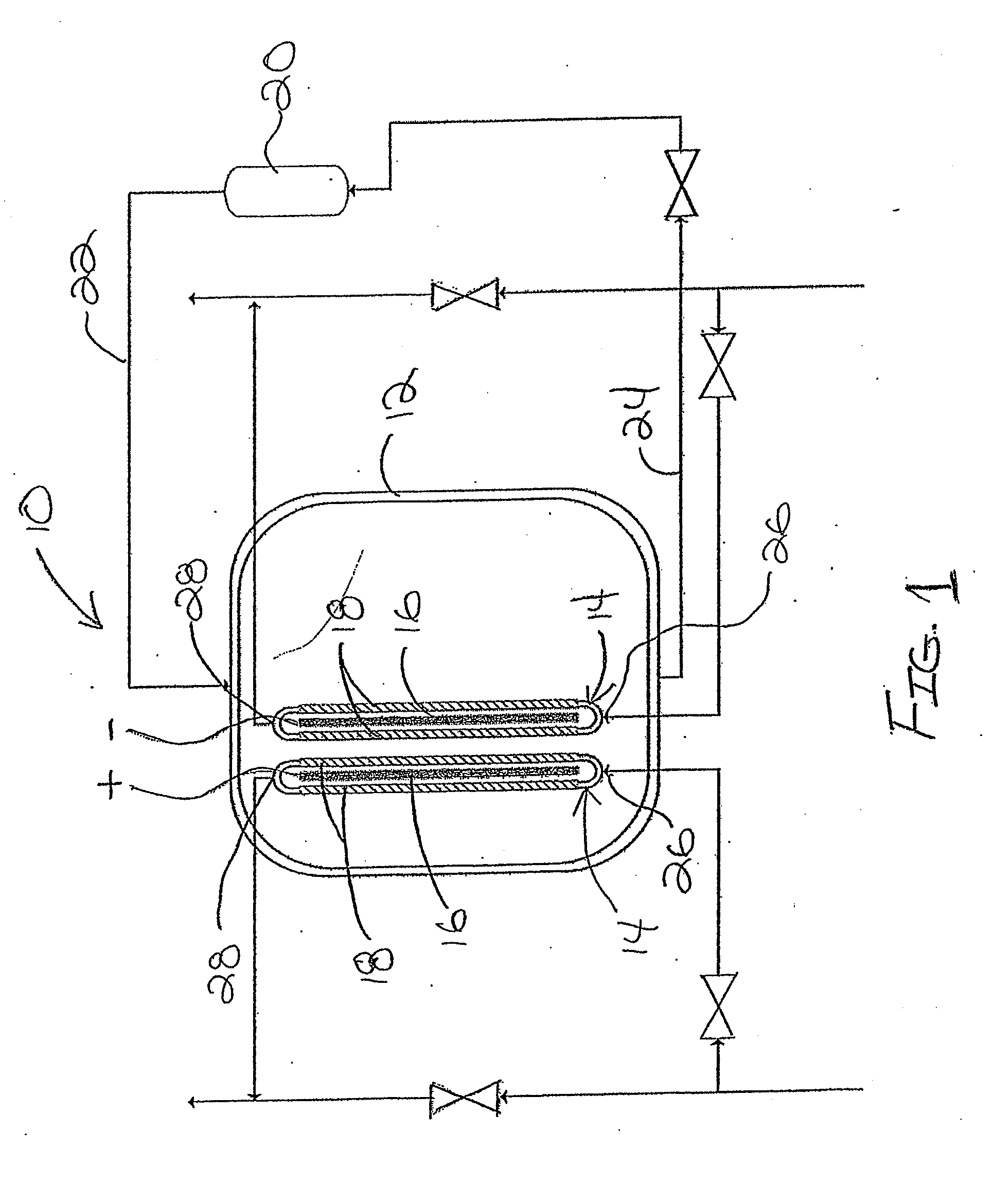
An electrolyzing system for electrolyzing a brine solution of water and an alkali salt to produce acidic electrolyzed water and alkaline electrolyzed water is provided. The system includes an internal chamber for receiving the brine solution and two electrolyzer cells immersed in a brine bath. Each electrolyzer cell includes an electrode, at least one ion permeable membrane supported relative to the electrode to define a space communicating between a fresh water supply and a chemical outlet into which brine enters only through the membrane. One of the electrodes is coupled to a positive charging electrical supply and the other to a negative charging electrical supply.
Owner:SPRAYING SYST
Semi-solid electrodes having high rate capability
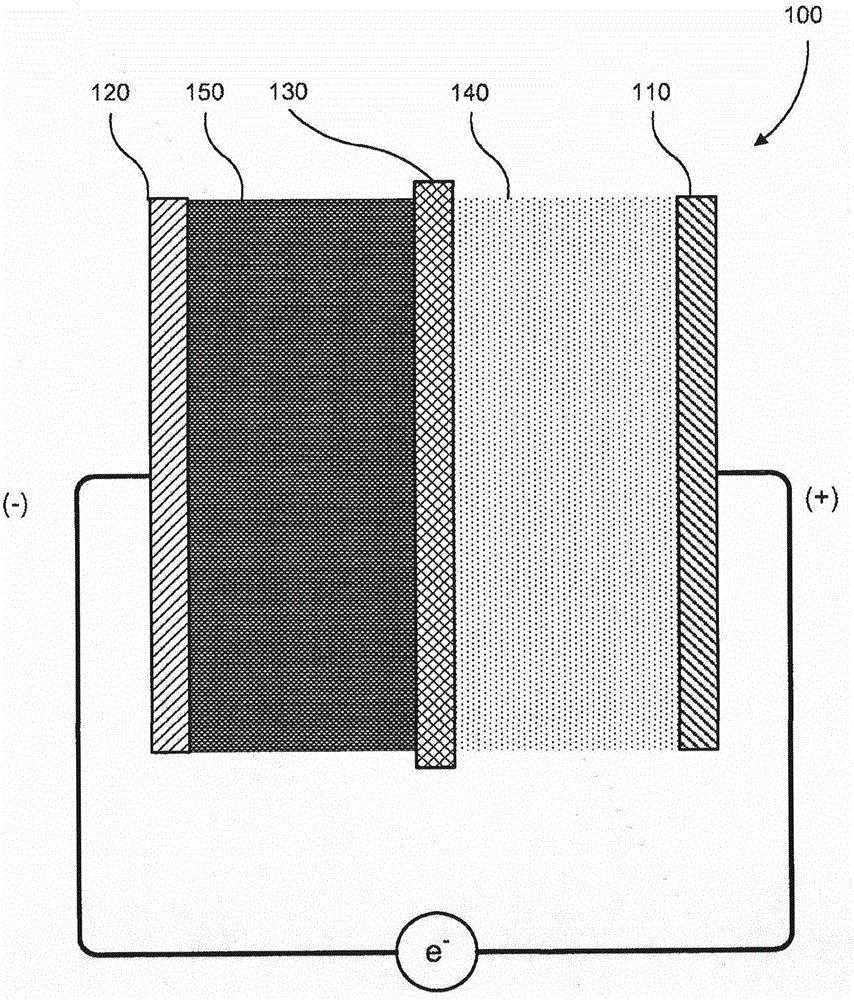
Embodiments described herein relate generally to electrochemical cells having high rate capability, and more particularly to devices, systems and methods of producing high capacity and high rate capability batteries having relatively thick semi-solid electrodes. In some embodiments, an electrochemical cell includes an anode, a semi-solid cathode that includes a suspension of an active material and a conductive material in a liquid electrolyte, and an ion permeable membrane disposed between the anode and the cathode. The semi-solid cathode has a thickness in the range of about 250 μm-2,500 μm, and the electrochemical cell has an area specific capacity of at least 5 mAh / cm2 at a C-rate of C / 2.
Owner:24M TECH INC
Power storage device and power storage system
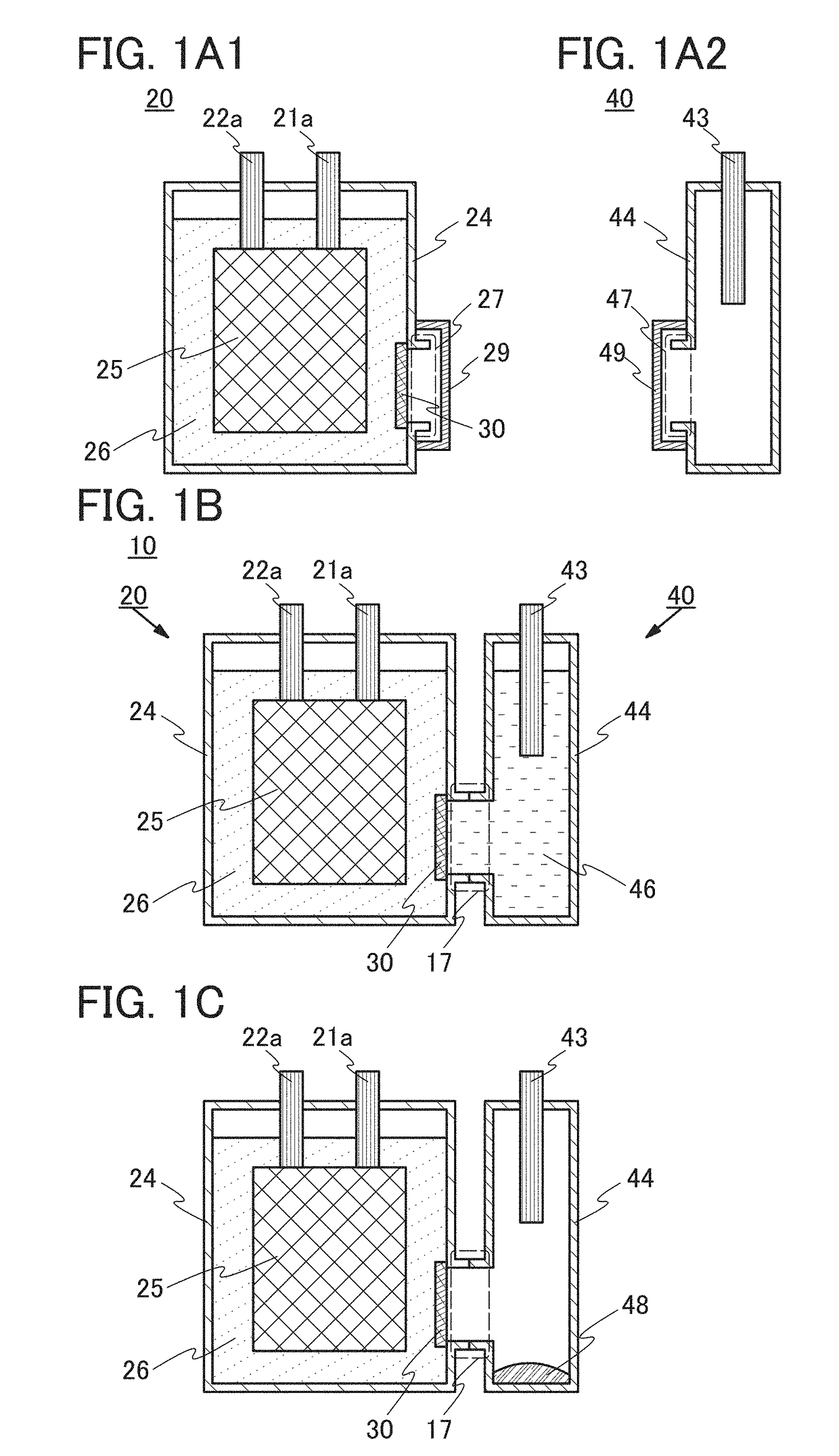
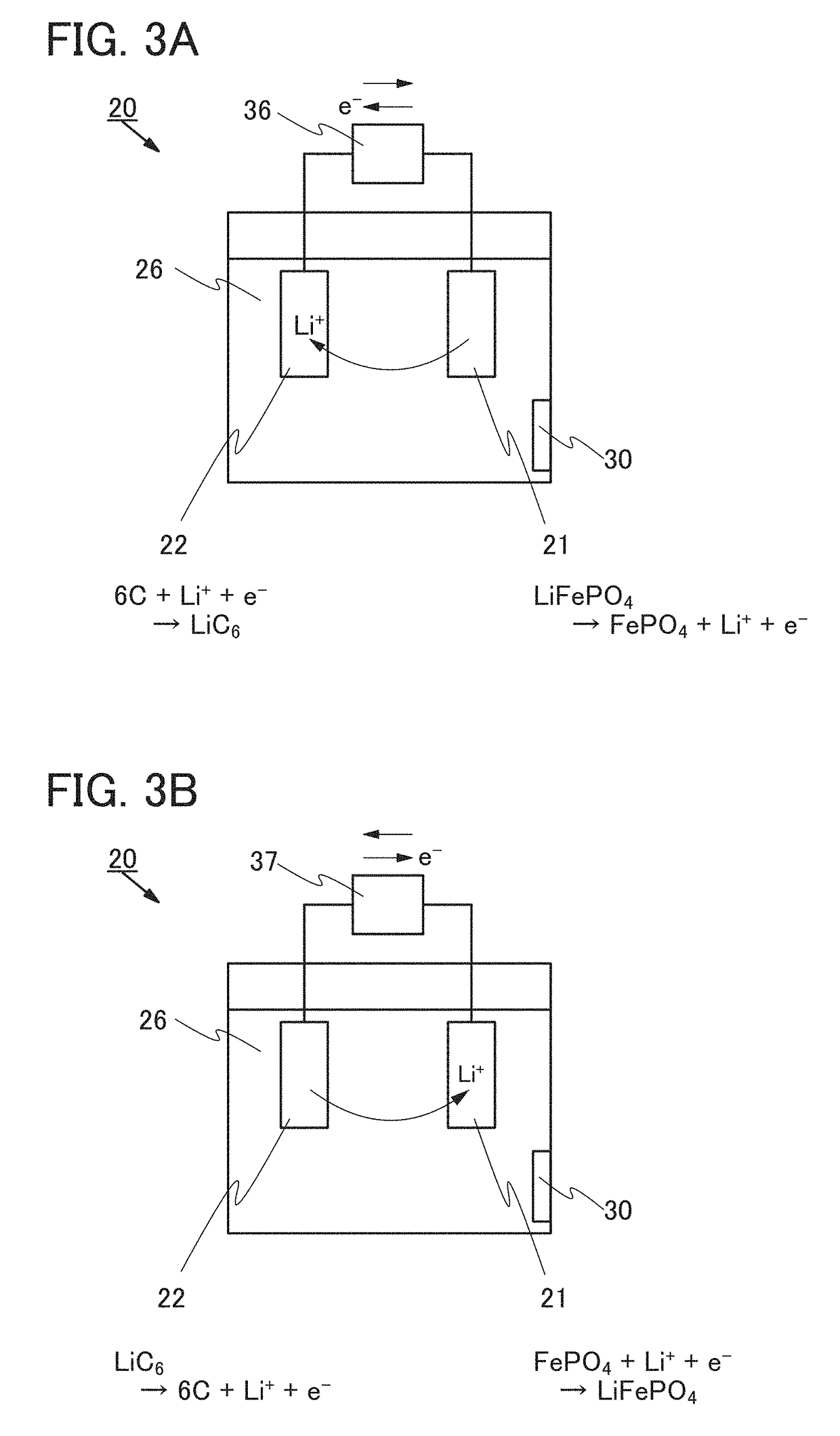
ActiveUS20170244135A1Reduce capacityElectrolyte moving arrangementsBatteries circuit arrangementsEngineeringElectric power
A power storage system or a power storage device that can restore reduced capacity is provided. The power storage device includes a first exterior body, a first electrode, a second electrode, a first electrolyte solution, and a carrier ion permeable film. The first electrode, the second electrode, and the first electrolyte solution are covered with the first exterior body. The first electrode and the second electrode are in contact with the first electrolyte solution. The first electrolyte solution includes carrier ions. A first opening is provided in the first exterior body. The carrier ion permeable film is provided to be in contact with the first electrolyte solution and so as to block the first opening without any space. The carrier ion permeable film is configured to be impermeable to water and air but permeable to the carrier ions.
Owner:SEMICON ENERGY LAB CO LTD
Cathodes capable of operating in an electrochemical reaction, and related cells, devices, and methods
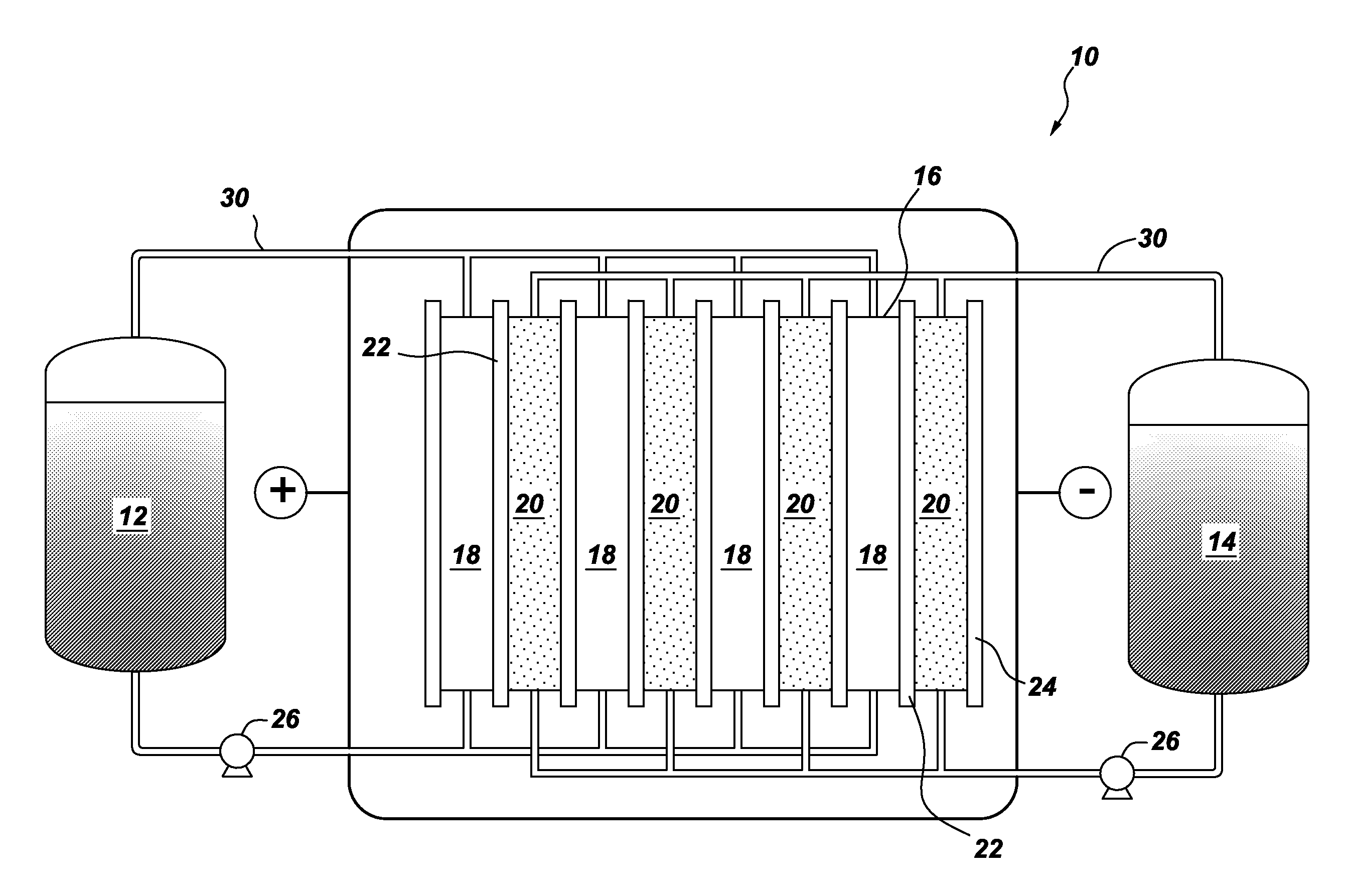
A flow battery is described, including a catholyte in the form of an aqueous solution of at least one salt of a halogen oxoacid compound; and an anolyte that includes an eletrochemically-active material capable of participating in a reduction-oxidation (redox) reaction with the catholyte salt; along with an intervening ion-permeable membrane. A unique cathode used in the battery or for other purposes is also described, along with a method of providing electrical energy to a device, system, or vehicle, using the flow battery.
Owner:GENERAL ELECTRIC CO
Electrolyzing system
Owner:SPRAYING SYST
Alkali-Ion Battery with Enhanced Transition Metal Cyanometallate Electrode Structure
ActiveUS20160056467A1Short assembly timeImprove throughputComplex cyanidesFinal product manufactureAlkali ionsAqueous solution
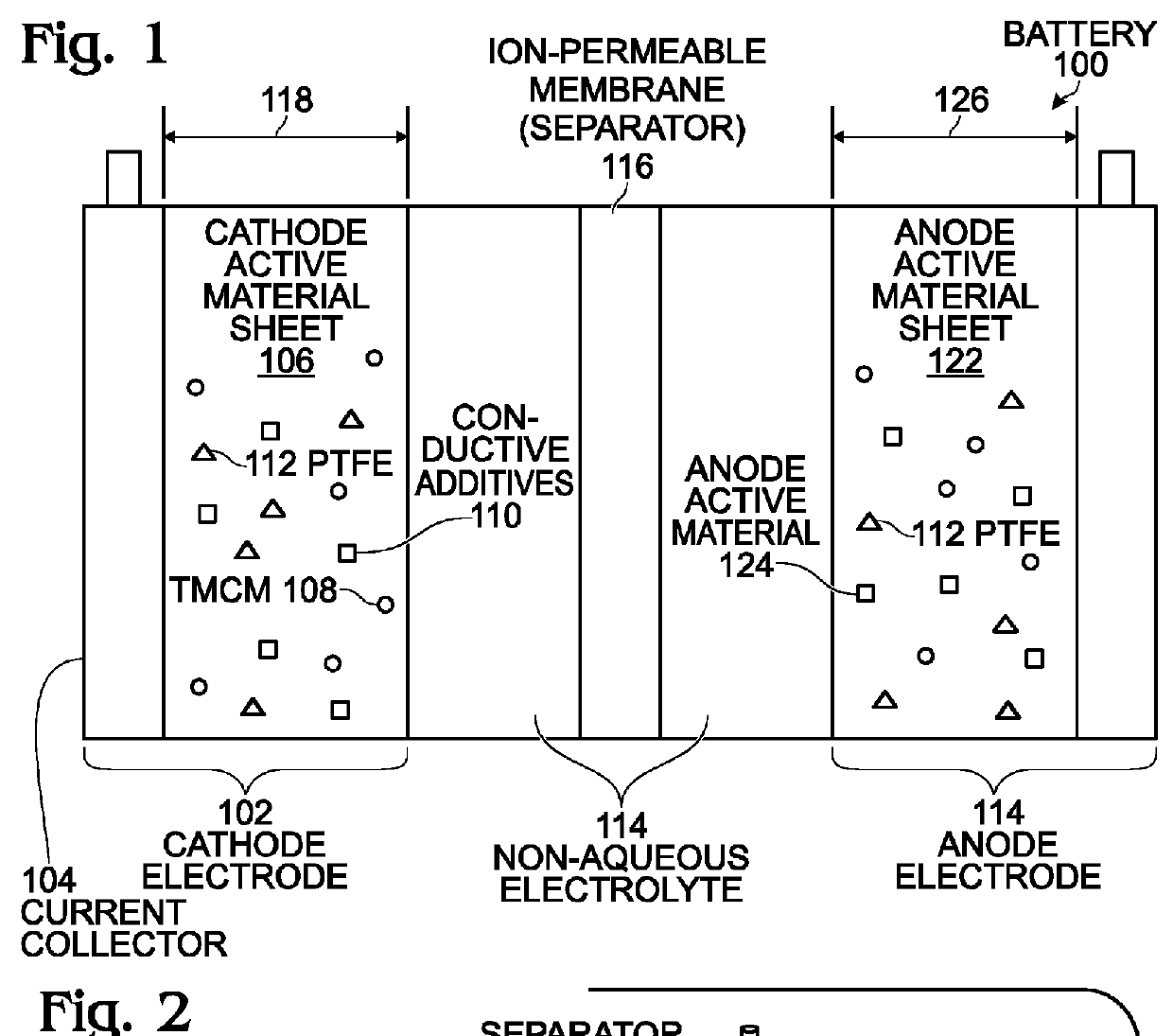
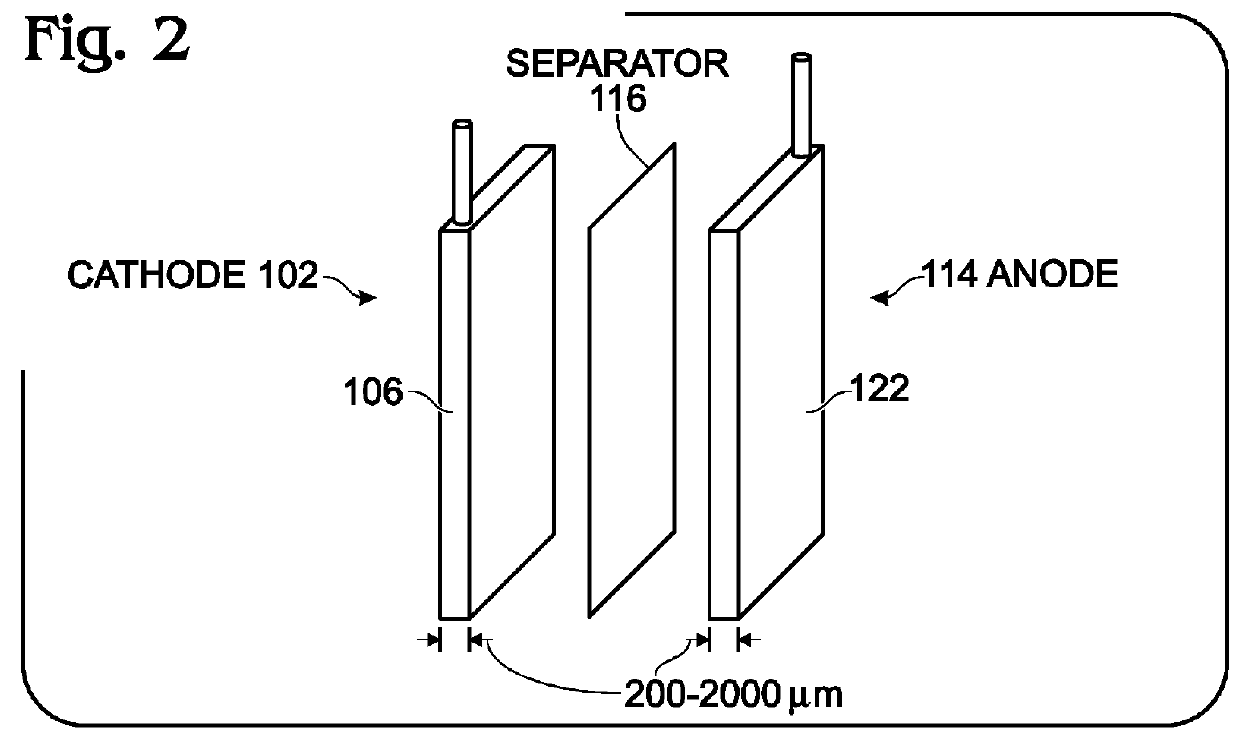
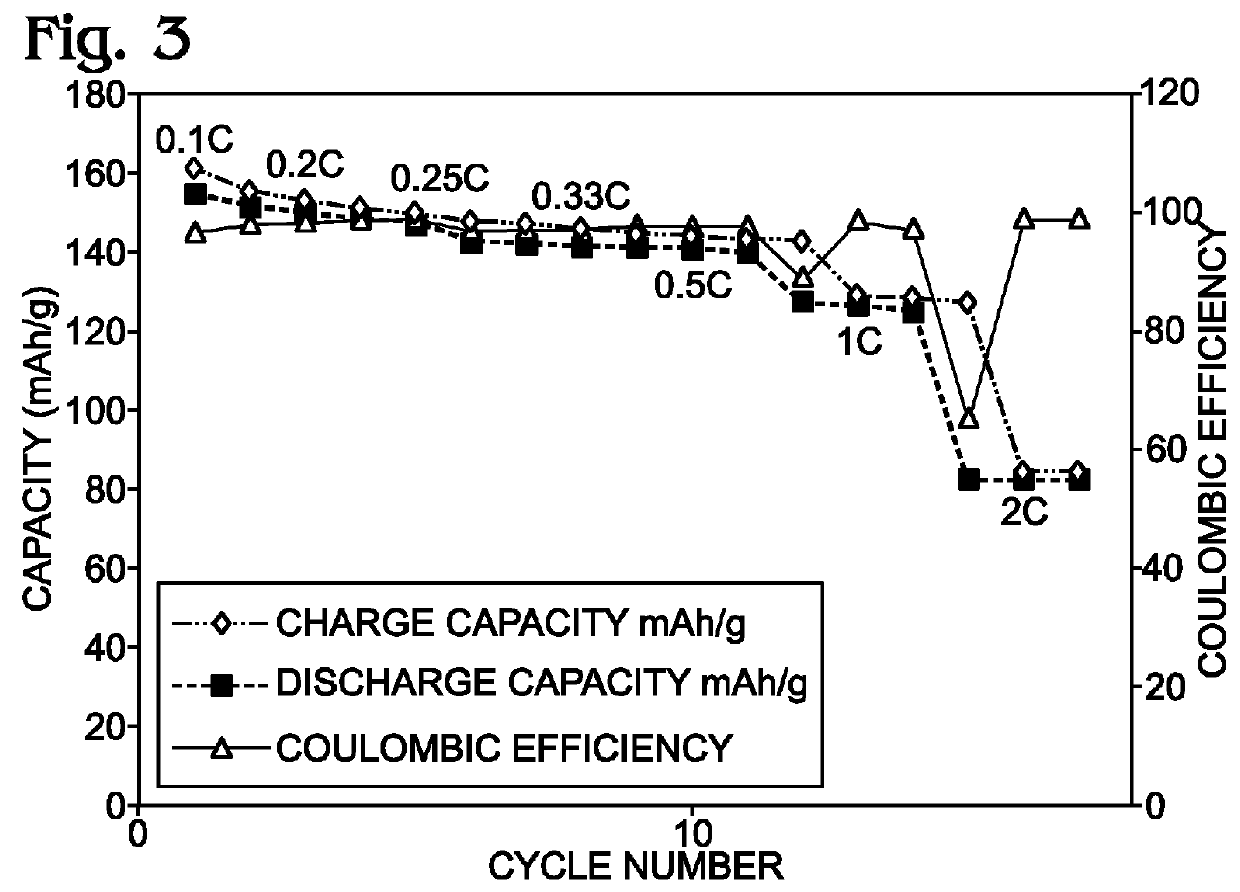
An alkali-ion battery is provided with a transition metal cyanometallate (TMCM) sheet cathode and a non-alkaline metal anode. The fabrication method mixes TMCM powders, conductive additives, and a polytetrafluoroethylene binder with a solution containing water, forming a wet paste. The wet paste is formed into a free-standing sheet of cathode active material, which is laminated to a cathode current collector, forming a cathode electrode. The free-standing sheet of cathode active material has a thickness typically in the range of 100 microns to 2 millimeters. The cathode electrode is assembled with a non-alkaline metal anode electrode and an ion-permeable membrane interposed between the cathode electrode and anode electrode, forming an assembly. The assembly is dried at a temperature of greater than 100 degrees C. The dried assembly is then inserted into a container (case) and electrolyte is added. Thick anodes made from free-standing sheets of active material can be similarly formed.
Owner:SHARP KK
Preparation method and application of supercapacitor based on ultrathin two-dimensional nickel hydroxide nano material
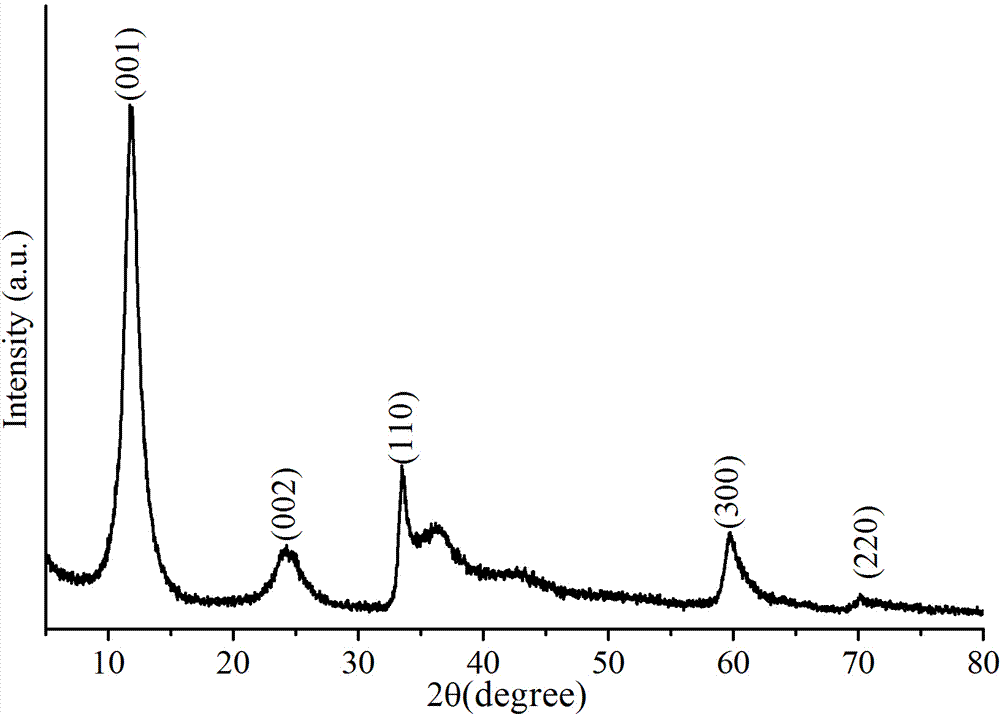
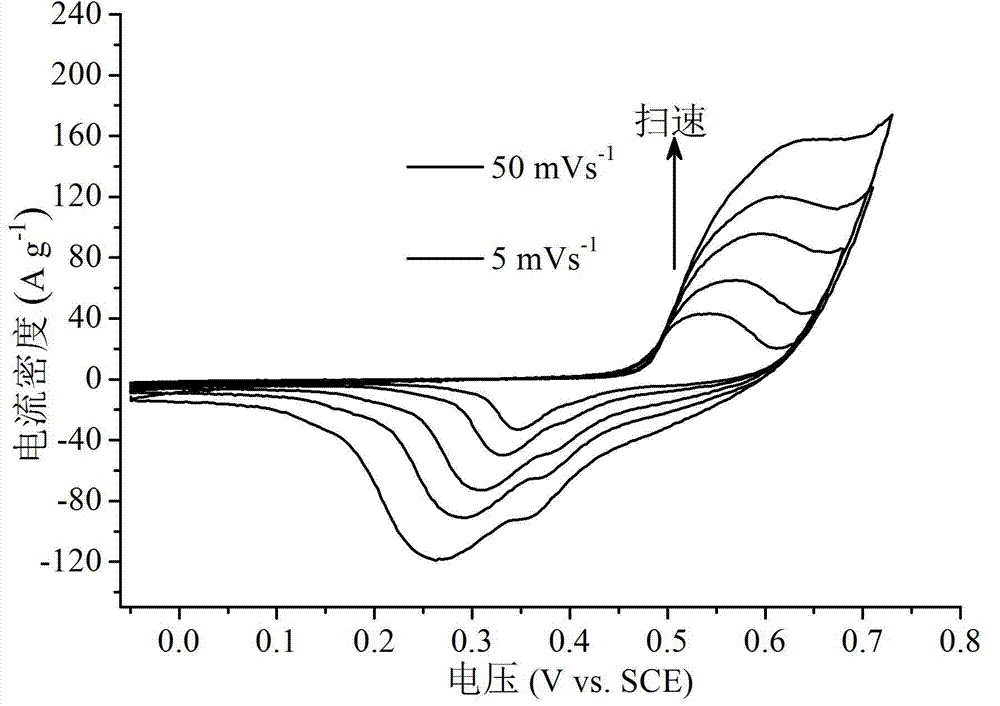
InactiveCN103489663AHigh specific capacityImproved magnification performanceHybrid/EDL manufacturePlatinumNew energy
The invention discloses a preparation method and application of a supercapacitor based on an ultrathin two-dimensional nickel hydroxide nano material. By taking the ultrathin two-dimensional nickel hydroxide nano material as an active substance, the preparation method of the supercapacitor comprises the following steps of dispersing the active substance, a conductive agent and a binder in a dispersing agent according to certain mass ratio, performing ultrasonic treatment, mixing uniformly, applying the mixture to a battery-grade current collector, and performing vacuum drying and tableting, thus preparing a supercapacitor electrode; and soaking the electrode in an electrolyte for more than 10 hours, performing activating treatment, and assembling to form an asymmetrical supercapacitor with a carbon material as a counter electrode and an ion permeable membrane as a diaphragm, or assembling to form an analog supercapacitor with a large-area platinum sheet as the counter electrode and a saturated calomel electrode as a reference electrode, or performing electrochemical performance testing investigation in an alkaline electrolyte. The prepared supercapacitor has ultrahigh specific capacity, good rate capability and long cycle life, and particularly can meet the general requirements of new energy electric automobiles, thereby being a supercapacitor with most application foreground.
Owner:曹传宝
Polyethylene ion permeable membrane and preparation method thereof
InactiveCN101601972AImprove wear resistanceImprove low temperature impact resistanceSemi-permeable membranesPore distributionAntioxidant
The invention relates to a polyethylene ion permeable membrane and a preparation method thereof, comprising the following materials by weight part: 30-35 parts of silicon dioxide, 10-15 parts of polyethylene, 1-1.5 parts of color batch, 50-55 parts of stuffing bulking agents, and 0.1-0.2 part of antioxidant; the materials are uniformly mixed in a material mixing system, the mixed material is fed to a double screw extrusion machine uniformly through a discharging machine, and the material is discharged after being pressurized and melted by the double screw extrusion machine; the discharged mixture is formed by a forming machine, the formed semi-finished polyethylene ion permeable membrane is arranged in an oil extractor which is filled with trichloroethylene, so as to carry out oil control to the permeable membrane; the permeable membrane after oil control is carried out is dried, surface-activated, rolled and post-processed for obtaining finished products. The method has the advantages of low electric resistance, small bore diameter, high aperture ratio, uniform pore distribution; the permeable membrane has strong anti-piercing performance, corrosion resisting performance and oxidation resistance, and the whole production process is clean without pollution.
Owner:JIANGSU EPCOH TECH
Methods and stabilized compositions for reducing deposits in water systems
InactiveCN104250827AWater treatment parameter controlElectrolysis componentsElectricityHypochlorous acid
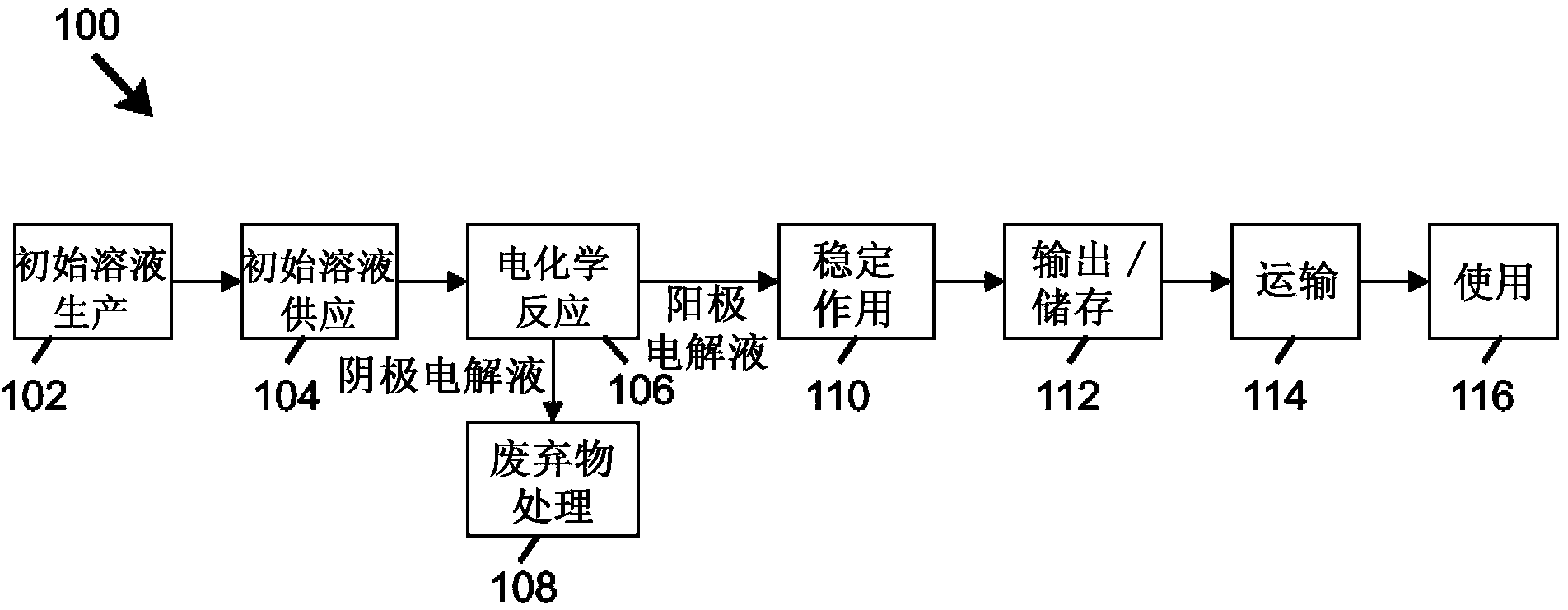
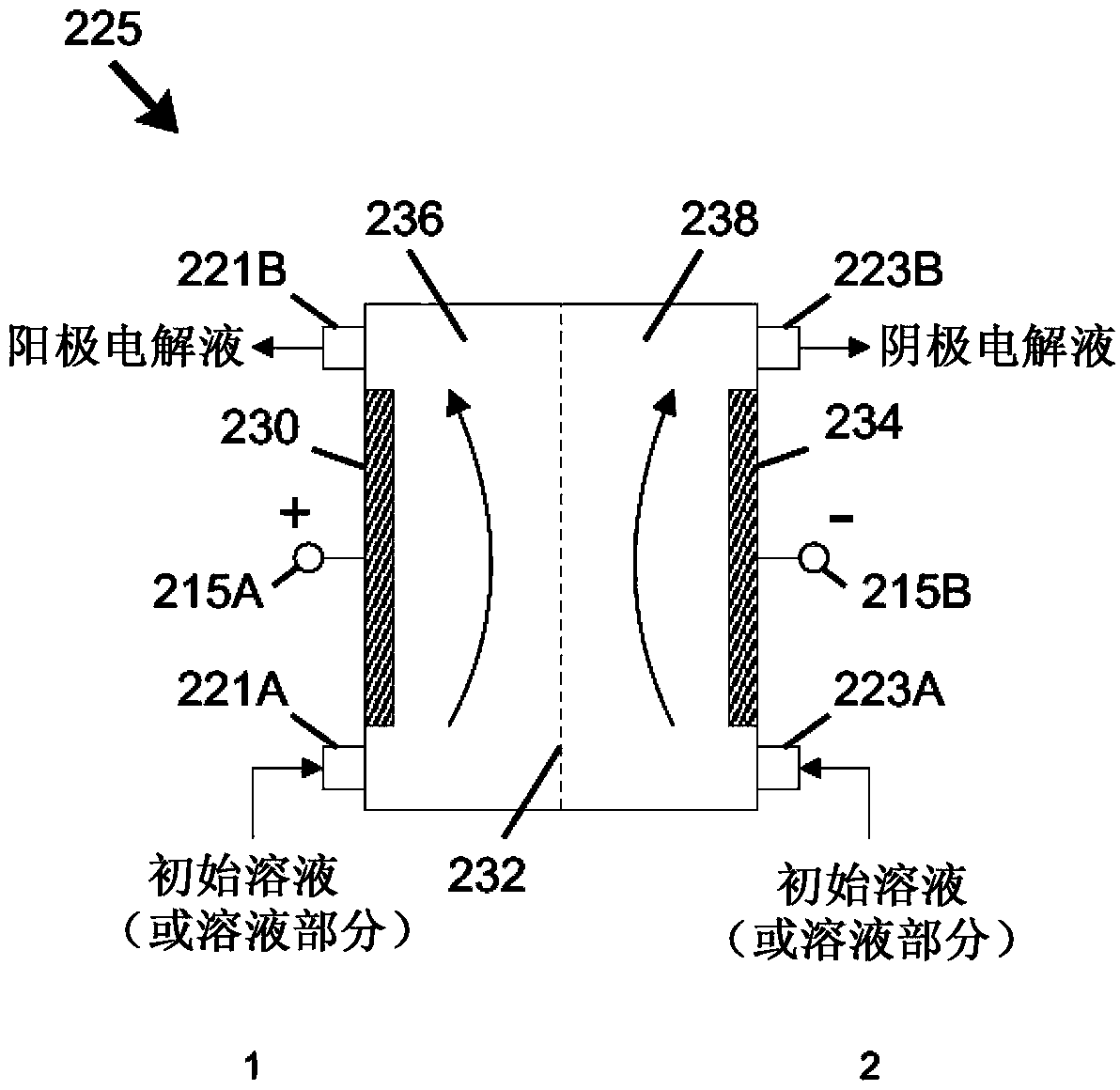
The invention relates to methods and stabilized compositions for reducing deposits in water systems. A stabilized mixed oxidant solution may be produced by flowing a starting solution (e.g., salt brine, hypochlorous acid, and / or sodium hypochlorite) through a flow-through electrochemical module including first and second passages separated by an ion permeable membrane while electric power is applied between an anode and cathode in electrical communication with the first and second passages, respectively. An initially acidic anolyte solution received from the first (anode) passage is stabilized by elevating pH to yield a stabilized mixed oxidant solution. Methods of using the mixed oxidant solution are further provided.
Owner:BLUE EARTH LABS
Electrolytic method for controlling the precipitation of alumina
InactiveCN101772468AImprove A/TCImprove sedimentation efficiencyAluminium compoundsElectrolysisElectric potential
A method for controlling the precipitation of alumina from a Bayer process solution, the method comprising the steps of: applying a potential between a first region comprising a Bayer process liquor and a second region comprising a caustic solution, wherein an ion permeable membrane is provided between the first region and the second region; and causing transfer of an ion across the ion permeable membrane from one region to the other region, wherein the Bayer process liquor is not directed to the second region.
Owner:ALCOA OF AUSTRALIA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com