Electrolysis vessel and apparatus for generating electrolyzed water
a technology of electrolysis vessel and electrolysis apparatus, which is applied in the direction of electrolysis components, chemistry apparatus and processes, water/sludge/sewage treatment, etc., can solve the problems of low electrolysis efficiency relative to applied power, inability to fully improve electrolysis efficiency relative to applied voltage, and increase in equipment siz
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
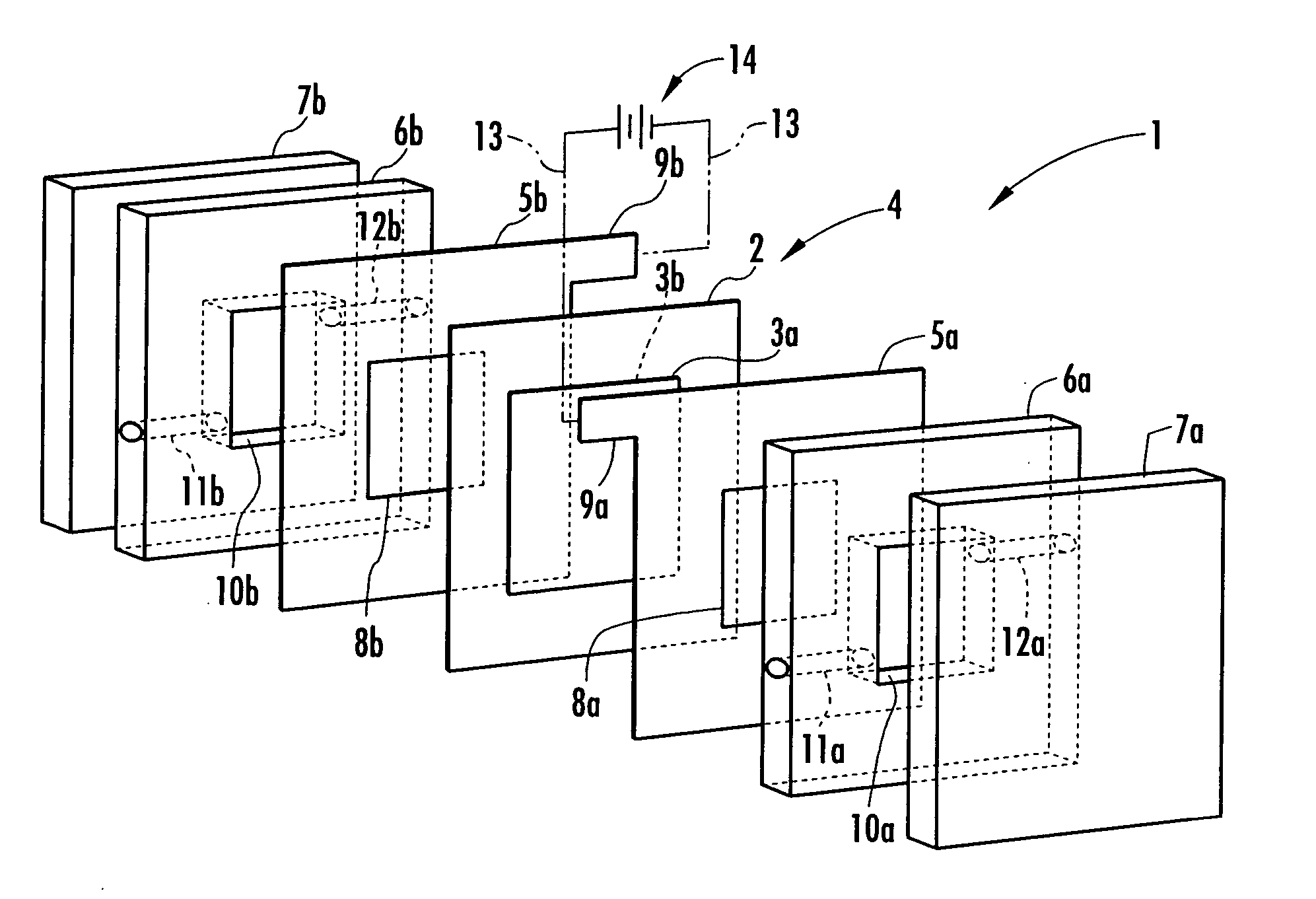
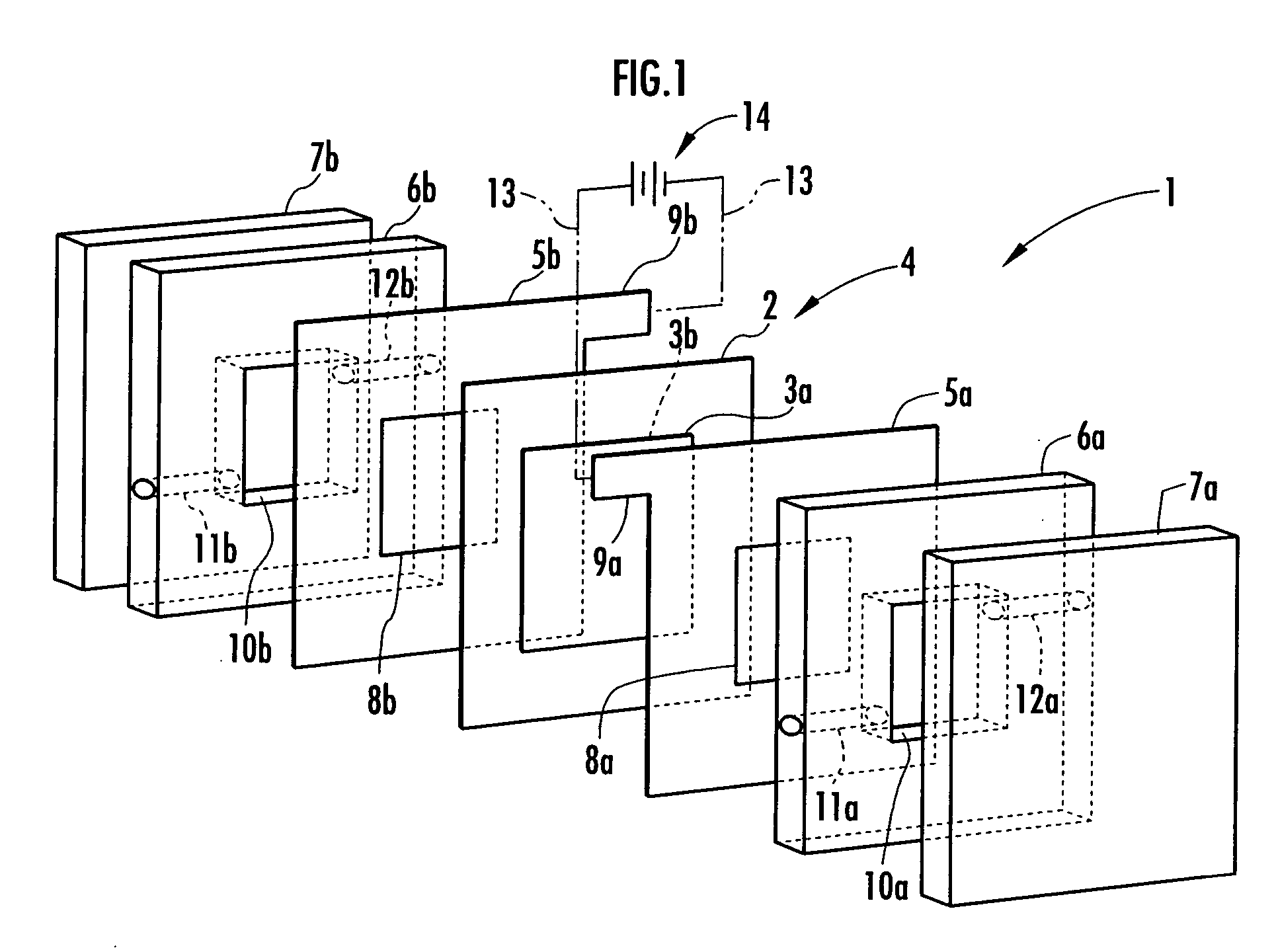
[0050] In this Example, the area of the electrodes 3a and 3b facing the electrolysis rooms 10a and 10b was set at 16 cm2. Distilled water and brine (aqueous solution of sodium chloride) having a concentration of 0.8 g / L were fed to the electrolysis room 10a on the anode side and the electrolysis room 10b on the cathode side, respectively each at a flow rate of 16 mL / min and a constant current of 0.5 A was applied to the electrodes 3a and 3b to effect electrolysis.
[0051] At the time, the voltage was approximately 7V, acidic electrolyzed water produced in the electrolysis room 10a had a pH of 1.94 and an effective chlorine concentration was 50 ppm. The results are shown in Table 1.
[0052] The above-described effective chlorine concentration was determined by adding 0.5 mL of a 0.1 mole / L silver nitrate solution dropwise to 5 mL of the acidic electrolyzed solution to form a white precipitate of silver chloride and measuring the amount of transmitted light of the turbid solution. The s...
example 2
[0061] In this Example, a paste mixture was prepared by mixing an electrode base material, a catalyst and a binder at a weight ratio of 100:5:7. Titanium carbide (TiC) of 325 mesh or less was used as the electrode base material, while a 3:7 (weight ratio) mixture of platinum black and iridium black was used as the catalyst. As the binder, a solution obtained by dissolving polyvinyl alcohol having a saponification ratio of 100% in a water / ethanol mixed solvent (volumetric ratio, 1:1) at a concentration of 2 wt. % was used. The paste mixture had a viscosity of from 15 to 25 cps.
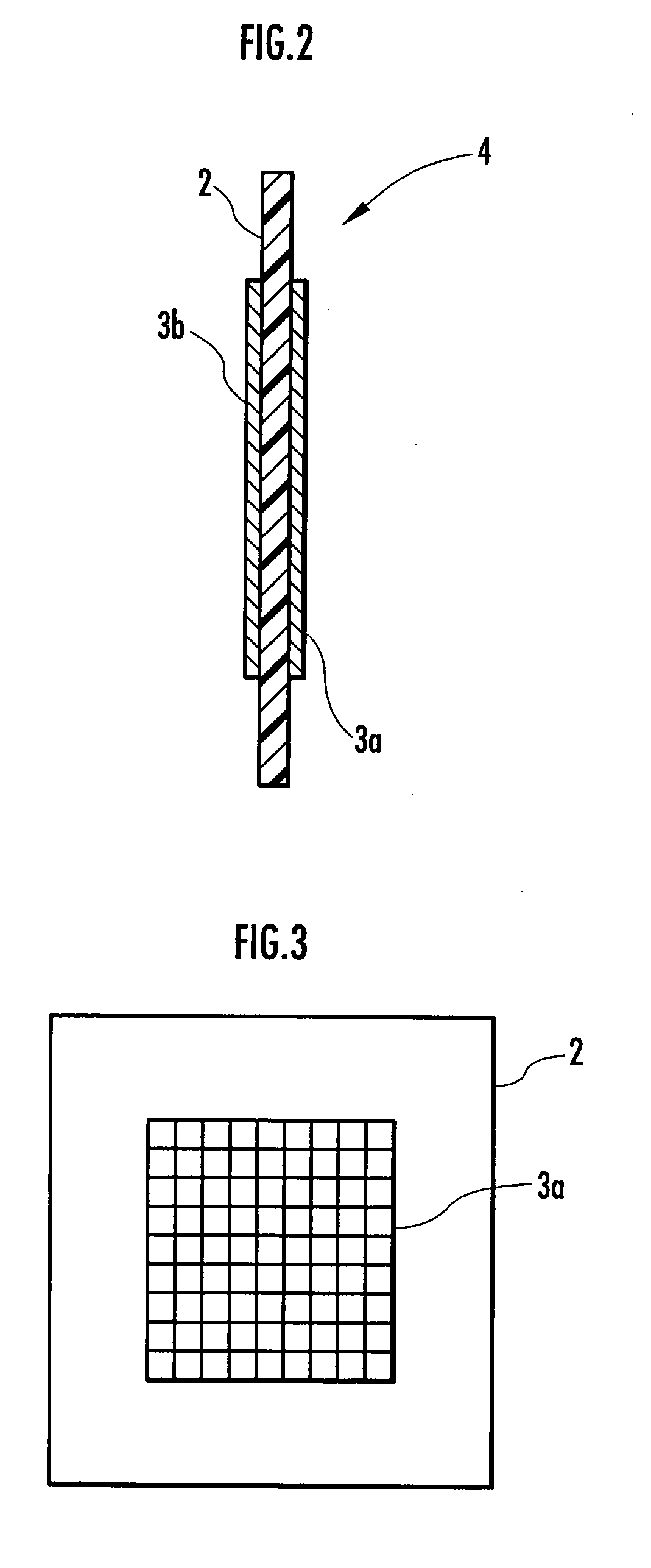
[0062] A membrane-electrode structure 4 was then formed by applying the paste mixture onto an anion exchange membrane (“Selemion (trade name) AMV”, product of Asahi Glass) having a thickness of about 100 μm, which was used as the ion permeable membrane 2, drying, and heating and pressurizing at 80° C. and 10 MPa for 30 minutes. As a result of measurement of the surface resistance of the electrolyzed water prod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Electrical conductor | aaaaa | aaaaa |
| Electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com