Benzylguanine derivatives and their organic salt compounds and pharmaceutical compositions and their applications
A technology of benzylguanine and derivatives, applied in the field of benzylguanine derivatives and their organic salt compounds and pharmaceutical compositions, can solve the problems of poor cell membrane permeability and low Pin1 affinity, and achieve easy preparation and druggability Good, promote biosynthesis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] A benzylguanine derivative (compound 1) provided in this example has a structural formula as shown below.
[0063]
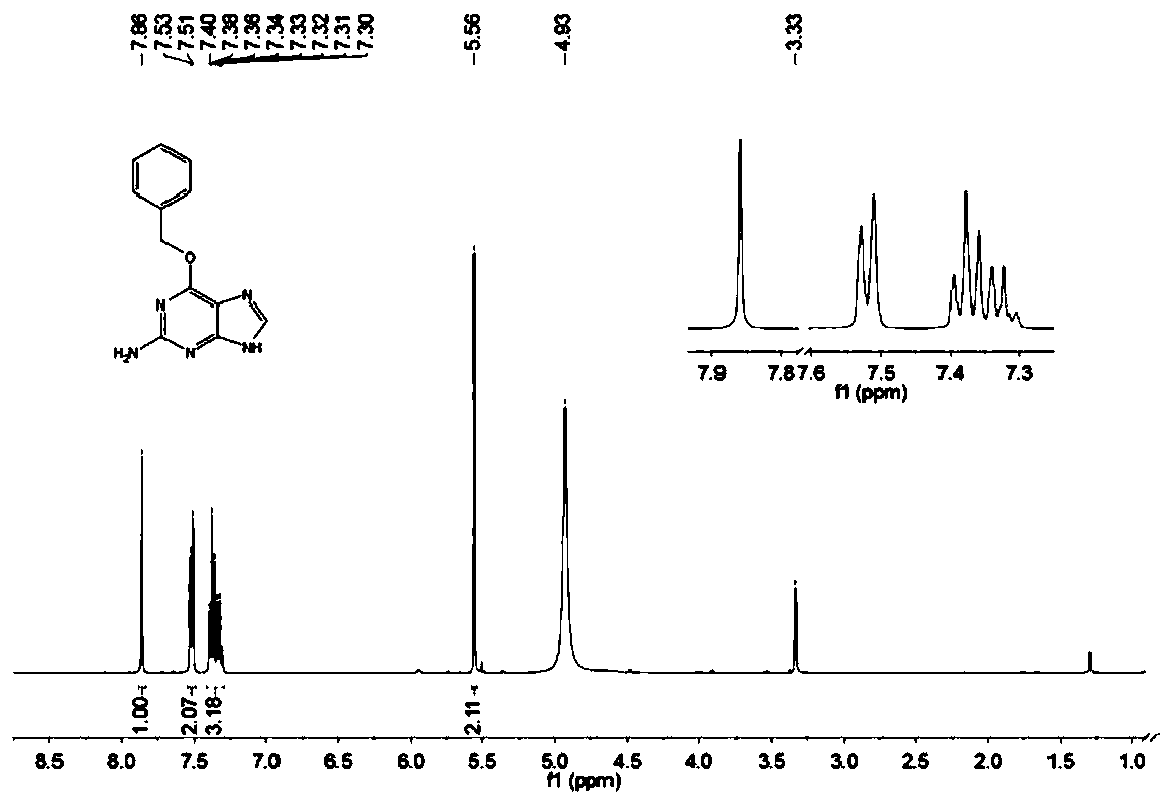
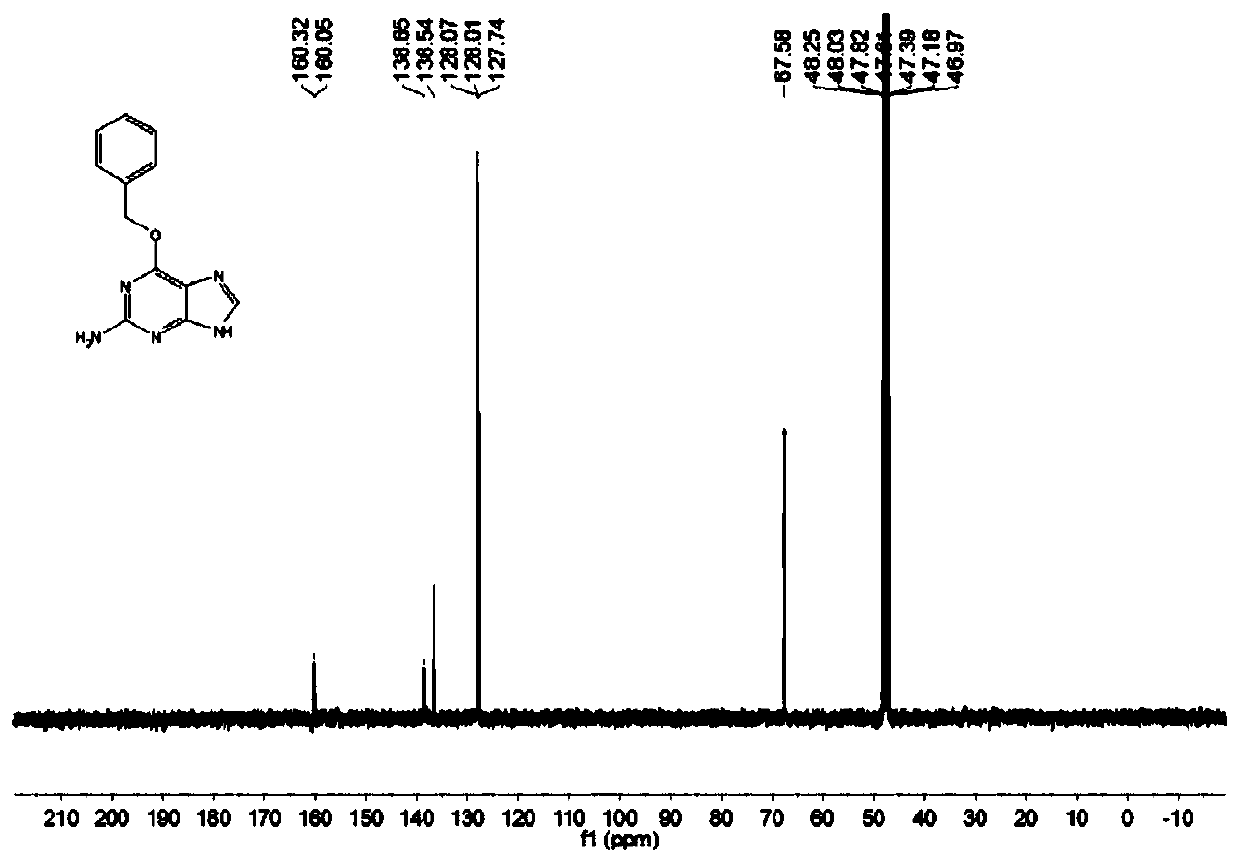
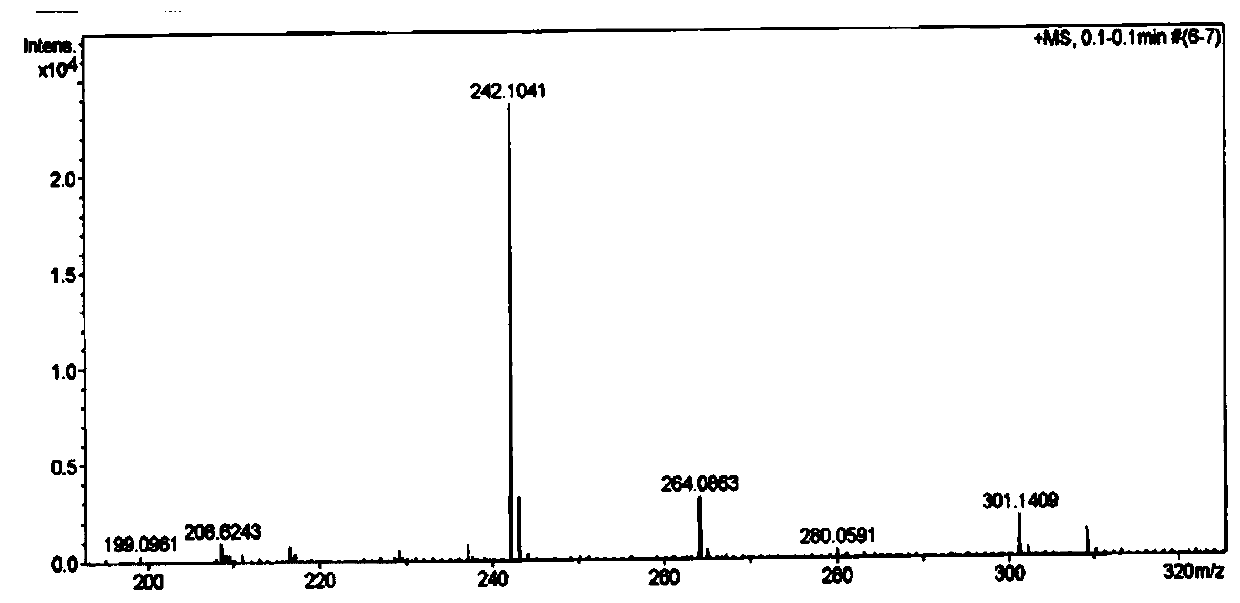
[0064] The structure of the benzylguanine derivative prepared by the embodiment of the present invention is passed 1 H NMR, 13 C NMR and HR-ESIMS for analytical determination. Specific map see figure 1 , figure 2 and image 3 . white solid. 1 H NMR (400MHz, Methanol-d 4 )δ7.86(s,1H), 7.52(d,J=7.2Hz,3H),7.42-7.29(m,4H),5.56(s,2H). 13 C NMR (100 MHz, Methanol-d 4 )δ160.32, 160.05, 153.83, 138.65, 136.54, 128.07, 128.01, 127.74, 118.32, 67.58.HR-ESIMS: 242.1041[M+H] + (calc.for C 12 h 12 N 5 O, 242.1037).
Embodiment 2
[0066] A benzylguanine derivative (compound 2) provided in this example has a structural formula as shown below.
[0067]
[0068] The structure of the benzylguanine derivative prepared by the embodiment of the present invention is passed 1 H NMR, 13 C NMR and HR-ESIMS for analytical determination. Specific map see Figure 4 , Figure 5 and Figure 6 . white solid. 1 H NMR (400MHz, DMSO-d 6 )δ12.49(s,1H), 7.86(s,1H),7.74-7.61(m,1H),7.59-7.51(m,1H),7.49-7.35(m,2H),6.34(s,2H) ,5.57(s,2H). 13 C NMR (100MHz, DMSO-d 6 )δ 162.22, 160.11, 156.54, 138.40, 134.67, 133.19, 130.88, 130.42, 129.82, 127.85, 114.46, 64.63. HR-ESIMS: 267.0650[M+H] + (calc.for C 12 h 11 ClN 5 O, 267.0647).
Embodiment 3
[0070] A benzylguanine derivative (compound 3) provided in this example has a structural formula as shown below.
[0071]
[0072] The structure of the benzylguanine derivative prepared by the embodiment of the present invention is passed 1 H NMR, 13 C NMR and HR-ESIMS for analytical determination. Specific map see Figure 7 , Figure 8 and Figure 9 . white solid. 1 H NMR (400MHz, DMSO-d 6 )δ12.53(s,1H), 7.87(s,1H),7.46(d,J=8.1Hz,2H),7.28(d,J=8.1Hz,2H),6.34(s,2H),5.45( s,2H),2.47(s,3H). 13 C NMR (100MHz, DMSO-d 6 )δ 161.43, 160.10, 156.54, 138.83, 138.53, 133.70, 129.78, 126.25, 120.05, 66.85, 15.13. HR-ESIMS: 268.0909[M+H] + (calc.for C 13 h 14 N 5 OS, 268.0913).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com