Achievement method of cation exchange of lead halide perovskite quantum dot material
A quantum dot material, cation exchange technology, applied in luminescent materials, chemical instruments and methods, nanotechnology, etc., to solve the problem of lead toxicity, high operability, and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
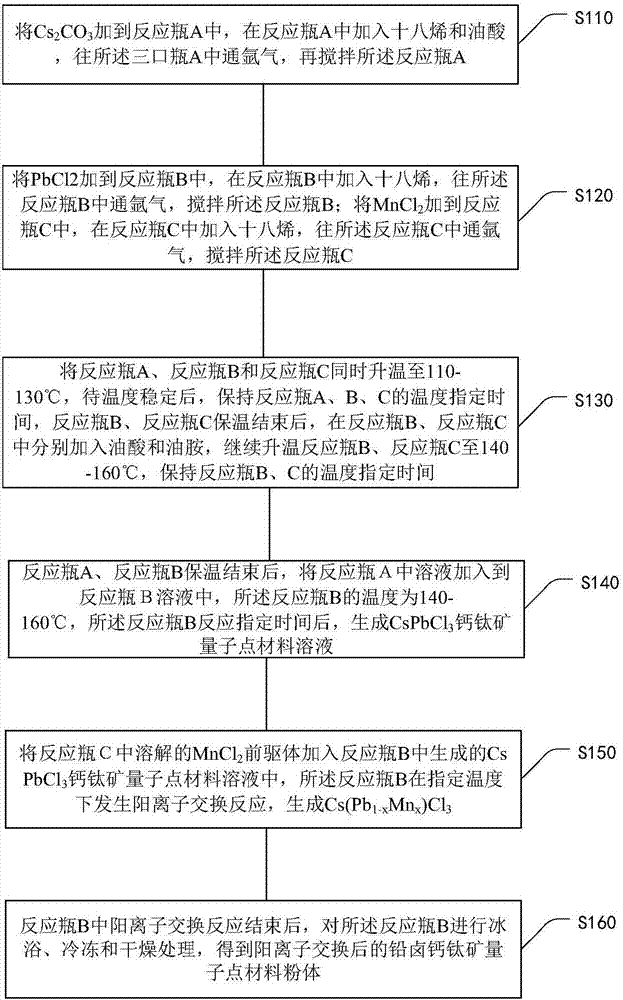
[0045] The processing flow of a method for realizing the cation exchange of a lead halide perovskite material proposed in the embodiment of the present invention includes the following processing steps:
[0046] Step 1, weigh Cs 2 CO 3 Put it into reaction bottle A, add octadecene and oleic acid in reaction bottle A, the volume ratio of octadecene and oleic acid is between 100:7 and 100:9, that is, greater than or equal to 100:7, less than or Equal to 100:9, the best ratio is 100:8. The different proportions of octadecene and oleic acid will seriously affect the shape and structure of the material. The shape, particle size and composition of the material system have very strict requirements on the parameters of the experiment.
[0047] Cs in reaction flask A after addition of octadecene and oleic acid 2 CO 3 The concentration is 0.02-0.03g / mL, argon gas is passed into the three-necked bottle A, and the reaction bottle A is stirred for 5-10 minutes; the argon gas in the rea...
Embodiment 2
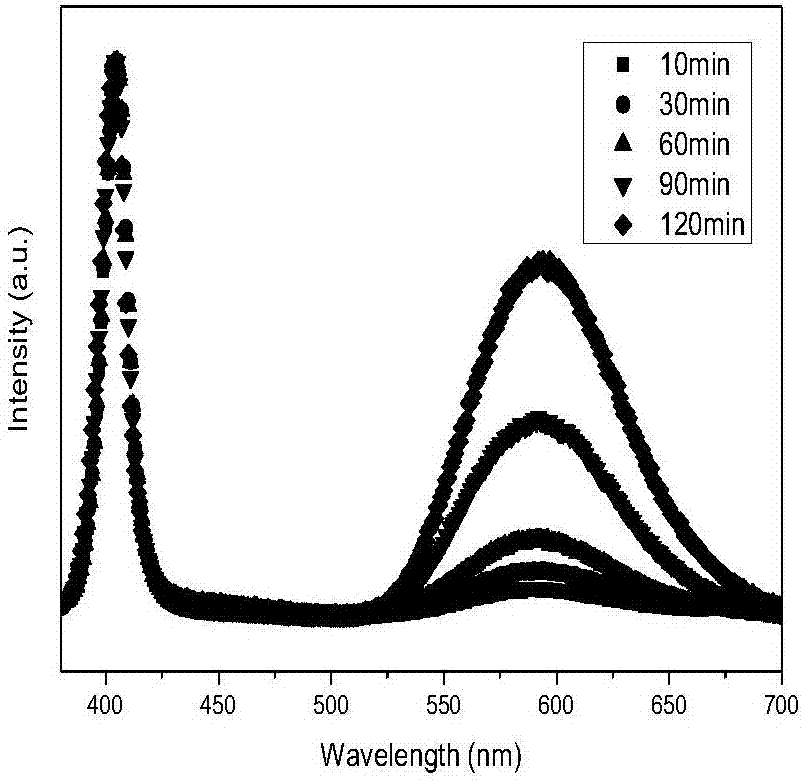
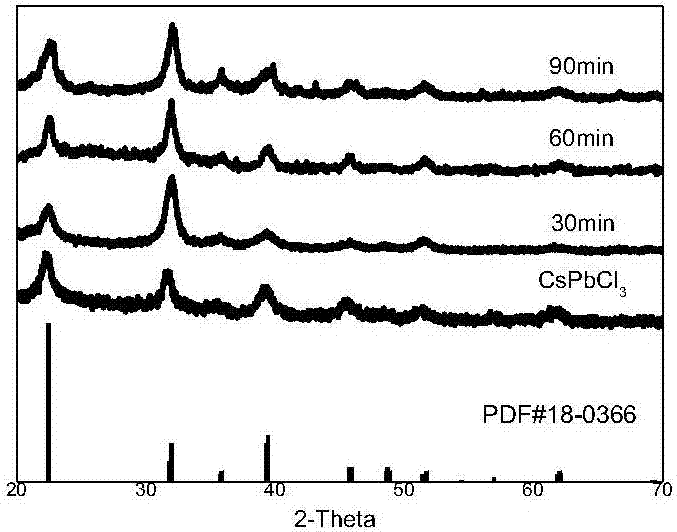
[0065] CsPbCl 3 and Mn 2+ Cation exchange reaction:
[0066] Step 1. Weigh 0.4g of Cs 2 CO 3 Put it into the three-necked bottle A, add 15mL of octadecene and 1.2mL of oleic acid, stir and pass through argon for 5min;
[0067] Step 2, 0.188mmol purity is 99.999% PbCl respectively 2 and MnCl 2 Add to 50mL three-neck flasks B and C, add 5mL of octadecene each, stir and pass through argon for 5min;
[0068] Step 3. Heat the three-neck bottles A, B, and C to 120°C at the same time, and keep the temperature for 30 minutes after the temperature stabilizes at 120°C;
[0069] Step 4. After the heat preservation is over, add 0.5mL of oleic acid and oleylamine to bottles B and C respectively, and continue to heat up to 150°C. 2 and MnCl 2 Fully dissolved respectively;
[0070] Step 5. After the heat preservation is over, take 0.6ml of the solution in bottle A and drop it into bottle B. After 10s, blue-emitting CsPbCl is generated. 3 Perovskite quantum dot materials;
[0071] ...
Embodiment 3
[0074] CsMnCl 3 and Pb 2+ Cation exchange reaction:
[0075] Step 1. Weigh 0.4g of Cs 2 CO 3 Put it into the three-neck flask A, add 15mL of octadecene and 1.2ml of oleic acid, stir and pass argon for 5min;
[0076] Step 2, 0.188mmol purity is 99.999% PbCl respectively 2 and MnCl 2 Add to 50mL three-neck flasks B and C, add 5mL of octadecene each, stir and pass through argon for 5min;
[0077] Step 3. Heat the three-neck bottles A, B, and C to 120°C at the same time, and keep the temperature for 30 minutes after the temperature stabilizes at 120°C;
[0078] Step 4. After the heat preservation is over, add 0.5mL of oleic acid and oleylamine to bottles B and C respectively, and continue to heat up to 150°C. 2 and MnCl 2 Fully dissolved respectively;
[0079] Step 5. After the heat preservation is over, take 0.6mL of the solution in bottle A and drop it into bottle C. After 10s, CsMnCl is generated 3 ;
[0080] Step 6, the PbCl dissolved in bottle B 2 The precursor is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com