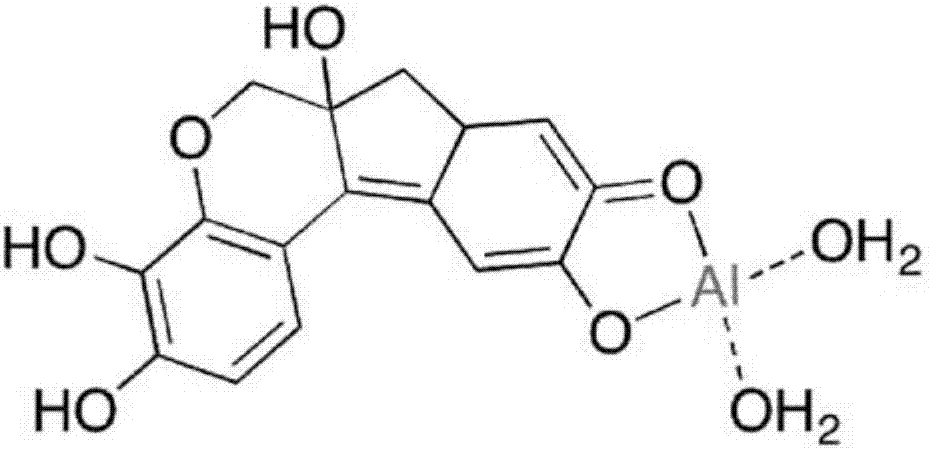
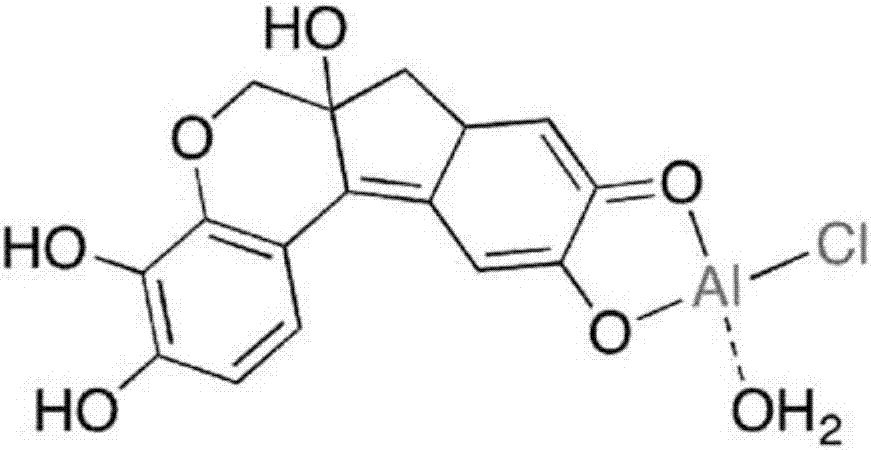
Hematoxylin solution comprising chloride and sulphate, and methods of preparation and use
A kind of technology of sulfate ion and hematoxylin, applied in the field of hematoxylin preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] ANS hematoxylin is based on the use of Al 2 (SO 4 ) 3 ·nH 2 O acts as a mordant and has been specifically optimized for automated H&E staining using a VentanaSYMPHONY autostainer. The formulations are based on those disclosed in US Patent 8,551,731. The hematoxylin preparation is prepared according to the following formulation:
[0062]
[0063] The composition is prepared according to the following procedure:
[0064] 1. Add / dissolve hematoxylin into a solution of water and glycol.
[0065] 2. Add / dissolve sodium iodate.
[0066] 3. Add / dissolve Al 2 (SO 4 ) 3 ·16H 2 o
[0067] 4. Add / dissolve hydroquinone and β-cyclodextrin
[0068] This composition is used as a benchmark for automated dyeing performance as it is the best known composition. The composition is capable of delivering proper staining on slides in about two minutes. While the composition stained well, with the correct shade and depth, and had sufficient long-term stability; it was not idea...
Embodiment 2
[0071] Prepare Cl with 1 / 0 - / SO 4 2- Molar ratio (i.e. 100% Cl - ) hematoxylin preparation. In addition to using AlCl 3 Hexahydrate instead of Al 2 (SO 4 )3 16H 2 Except for O, the same components and concentrations as in Example 1 were used.
[0072]
[0073] The composition was observed to be very stable, but also had very slow kinetics during dyeing. Therefore, the staining is very light under normal operating conditions.
Embodiment 3
[0075] Prepare Cl with 1 / 1 - / SO 4 2- Molar ratio (ie 50% Cl - ) hematoxylin preparation. In such formulations, including SO 4 2- , because it is completely removed from ANS-Cl, resulting in poor staining quality (depth and shade of staining) and unacceptable kinetics (eg too slow for automated staining). In addition, the concentration of hematoxylin was also increased to increase the kinetics, which is thought to be influenced by Cl - suppressed by the existence of
[0076]
[0077] The formulations showed high quality dyeing and adequate dyeing rates. After several experiments, it was concluded that higher concentrations of hematoxylin compared to ANS could be used to ensure staining kinetics matched the ANS benchmark. The compositions were observed to be sufficiently stable over extended periods of time without including β-cyclodextrin or hydroquinone included in the ANS. It was also observed that this formulation had a significantly delayed onset of precipitati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com