A nano-targeted drug carrier, preparation method and application thereof
A targeting and carrier technology, applied in pharmaceutical formulations, drug combinations, anti-tumor drugs, etc., can solve the problems that anti-tumor drugs do not have tumor tissue-targeting effects, affect drug bioavailability, and cannot achieve anti-tumor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
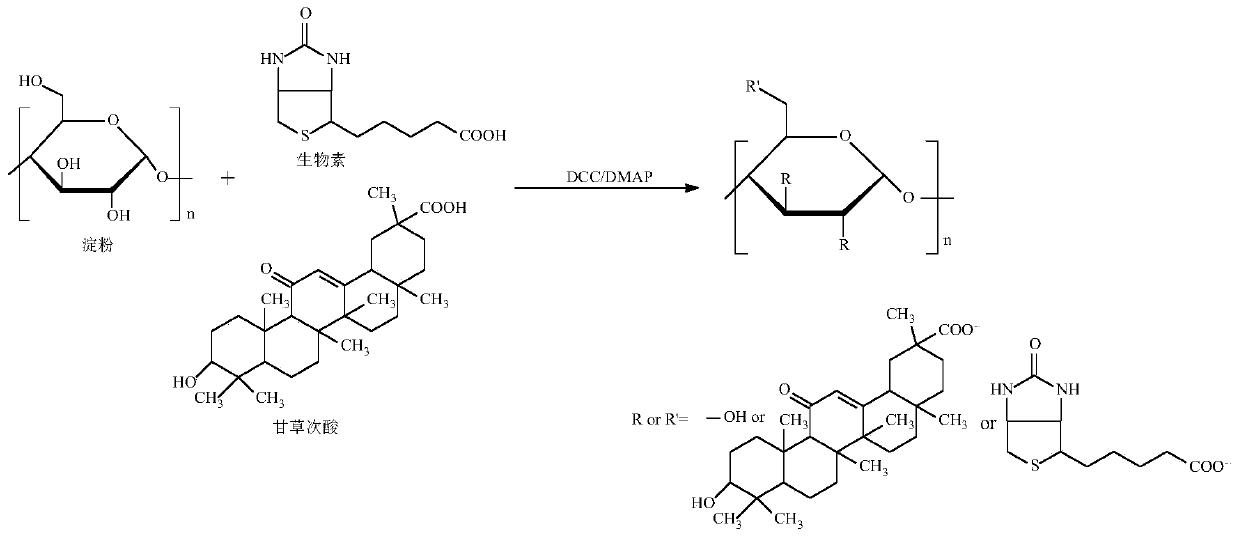
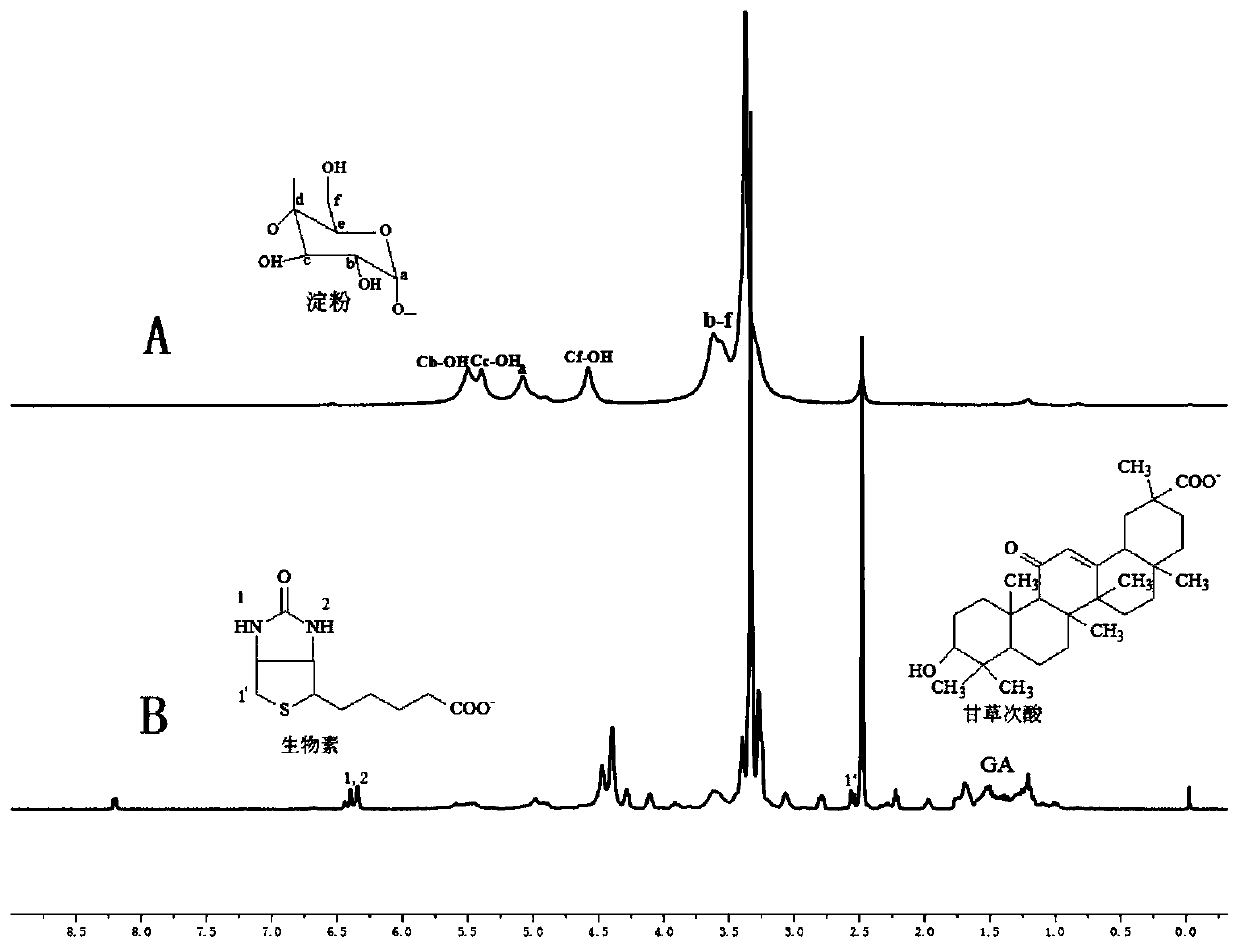
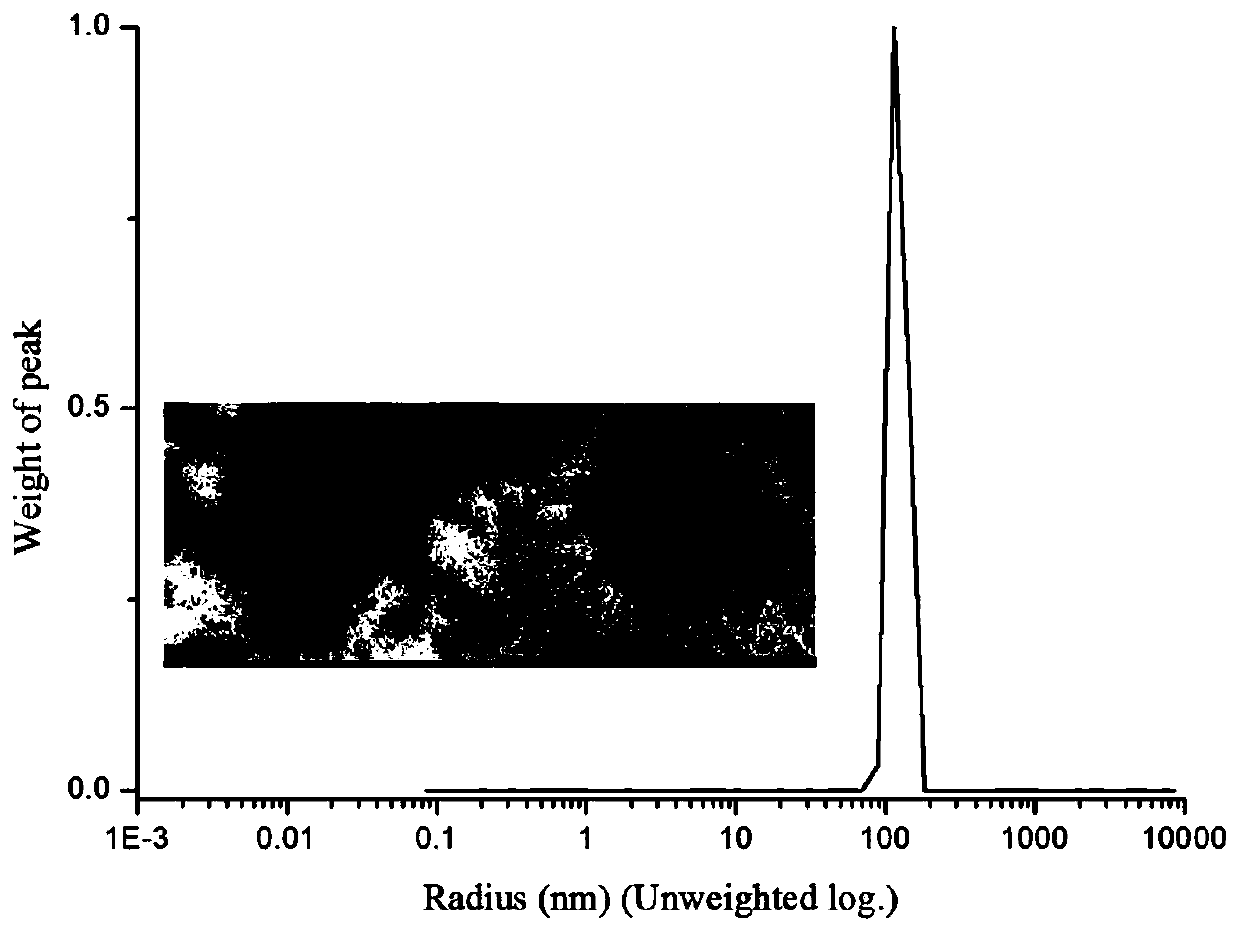
[0031] Experimental principle such as figure 1 , the specific preparation method is as follows:
[0032] a) Reaction step: get 3.0g soluble starch (polymerization degree is 400-800, relative molecular weight is 10 4 -10 6 g / mol), 1.5g biotin and 0.75g 18-β glycyrrhetinic acid were dissolved in 150mL dimethyl sulfoxide and stirred to dissolve, then 0.5g N,N'-dicyclohexylcarbodiimide and 0.25g 4- After dimethylaminopyridine was reacted at room temperature at a stirring speed of 150 rpm for 48 hours, the initial product was obtained;
[0033] b) Dialysis process: put the initial product prepared in the reaction step a) into a dialysis bag (MW8000-14000) and put it into deionized water for dialysis, change the deionized water every 4 hours, continue dialysis for 24 hours, and suction filter. And wash with anhydrous ether and deionized water 3 times respectively to obtain the washings, repeat the above steps 3 times, vacuum freeze-dry the washings for the third time at -50°C to ...
Embodiment 2
[0043] a) Reaction step: get 2.75g soluble starch (degree of polymerization is 400-800, relative molecular weight is 10 4 -10 6 g / mol), 1.25g biotin and 0.63g 18-β glycyrrhetinic acid were dissolved in 150mL DMSO and stirred to dissolve, then 0.6g N,N'-dicyclohexylcarbodiimide and 0.25g 4- After dimethylaminopyridine was reacted at room temperature at a stirring speed of 150 rpm for 48 hours, the initial product was obtained;
[0044] b) Dialysis process: put the initial product prepared in the reaction step a) into a dialysis bag (MW8000-14000) and put it into deionized water for dialysis, change the deionized water every 4 hours, continue dialysis for 24 hours, and suction filter. And wash with anhydrous ether and deionized water 3 times respectively to obtain the washings, repeat the above steps 3 times, vacuum freeze-dry the washings for the third time at -50°C to constant weight, and obtain glycyrrhetinic acid-modified biotin hydrophobic Modified soluble starch;
[004...
Embodiment 3
[0047] a) Reaction step: get 2.5g soluble starch (polymerization degree is 400-800, relative molecular weight is 10 4 -10 6 g / mol), 1.75g biotin and 0.87g 18-β glycyrrhetinic acid were dissolved in 150mL dimethyl sulfoxide and stirred to dissolve, then added 0.75g N,N'-dicyclohexylcarbodiimide and 0.25g 4- After dimethylaminopyridine was reacted at room temperature at a stirring speed of 150 rpm for 48 hours, the initial product was obtained;
[0048] b) Dialysis process: put the initial product prepared in the reaction step a) into a dialysis bag (MW8000-14000) and put it into deionized water for dialysis, change the deionized water every 4 hours, continue dialysis for 24 hours, and suction filter. And wash with anhydrous ether and deionized water 3 times respectively to obtain the washings, repeat the above steps 3 times, vacuum freeze-dry the washings for the third time at -50°C to constant weight, and obtain glycyrrhetinic acid-modified biotin hydrophobic Modified solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com