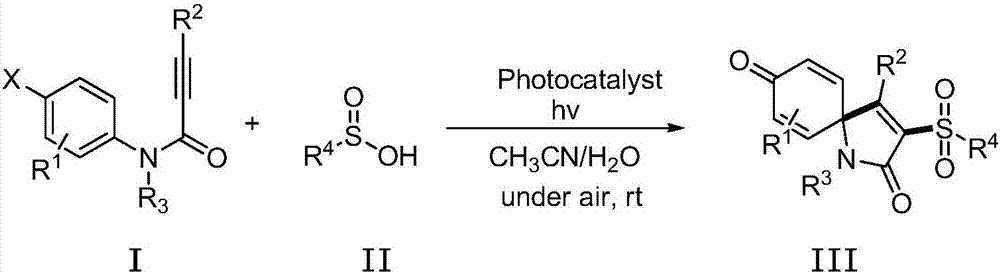
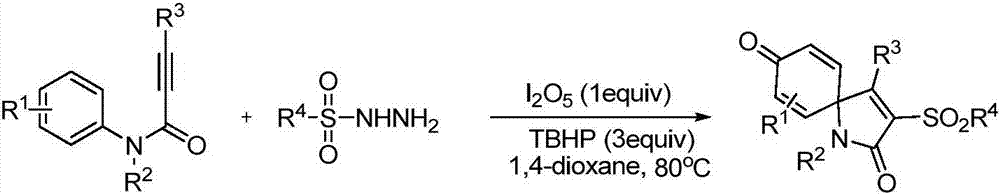Method for visible light catalytic synthesis of 3-sulfuryl spiro-trienone compound
A technology for sulfone-based spirocyclic trienone and compound, which is applied in the field of photocatalytic synthesis of 3-sulfone-based spirocyclic trienone compound, can solve problems such as potential safety hazards, restricted application and the like, achieves simple operation, stable reaction raw materials, cleanliness energy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
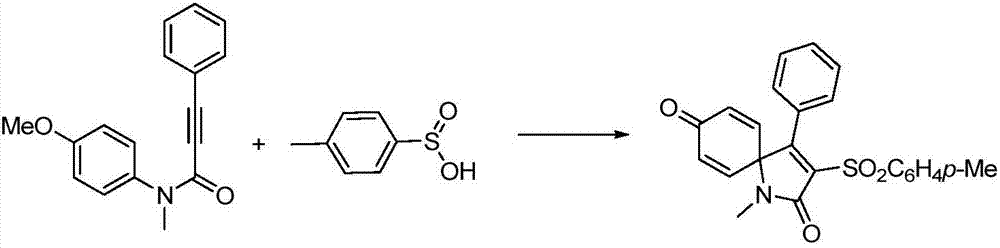
[0027] At room temperature, 33.1 mg of N-(4-methoxyphenyl)-N-methyl-3-phenylpropynamide and 58.5 mg of p-toluenesulfinic acid were successively added to a 15 mL reaction tube, and the photocatalyst was dissolved in water Eosin 4.3mg and acetonitrile and water (1:1) mixed solvent 2ml, mix well. Then under the irradiation of a 3w blue LED light, the reaction was stirred for 6h, and after the completion of the reaction was detected by TLC, it was concentrated under reduced pressure in a vacuum (0.08Mpa) to no solvent to obtain the crude product, and then the volume ratio was 2:1. Petroleum ether and Rinse with mixed eluents of ethyl acetate and perform flash column chromatography on a silica gel column to obtain the 3-sulfone spirotrienone product of this example as a white solid of 38.7 mg with a yield of 76%.
[0028] 1 H NMR (CDCl 3 ,500MHz,ppm):δ7.93(d,J=8.3Hz,2H),7.44(t,J=7.5Hz,1H),7.39-7.33(m,4H),7.15(d,J=7.2Hz, 2H), 6.43(s, 4H), 2.83(s, 3H), 2.44(s, 3H); 13...
Embodiment 2
[0030]
Embodiment 3
[0034]
[0035] At room temperature, 33.1 mg of N-(4-methoxyphenyl)-N-methyl-3-phenylpropynamide, 66.0 mg of p-chlorobenzenesulfinic acid, 4.3 mg, acetonitrile and water (1:1) mixed solvent 2ml, mix well. Then under the irradiation of a 3w blue LED lamp, the reaction was stirred and reacted for 10h. After the reaction was detected by TLC, it was concentrated to no solvent through vacuum (0.08Mpa) under reduced pressure to obtain the crude product, and then the volume ratio was 2:1. Petroleum ether and Rinse with mixed eluents of ethyl acetate and perform flash column chromatography on a silica gel column to obtain the 3-sulfone spirotrienone product of this example as 29.3 mg of a yellow solid with a yield of 55%.
[0036] 1 H NMR (CDCl 3 ,500MHz,ppm):δ7.99(d,J=8.7Hz,2H),7.52(d,J=8.7Hz,2H),7.46(t,J=7.5Hz,1H),7.39(t,J= 7.9Hz, 2H), 7.15(d, J=7.1Hz, 2H), 6.47-6.42(m, 4H), 2.84(s, 3H); 13 C NMR (CDCl 3 ,125MHz,ppm):δ183.0,163.4,162.4,142.0,141.3,137.5,136.4,134.5,130.8,130...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com