Preparation method of folic acid
A folic acid, acid-soluble technology, applied in the field of medicine, can solve the problems of large water consumption and waste water, and achieve the effects of high comprehensive yield, cost reduction, and waste water volume reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
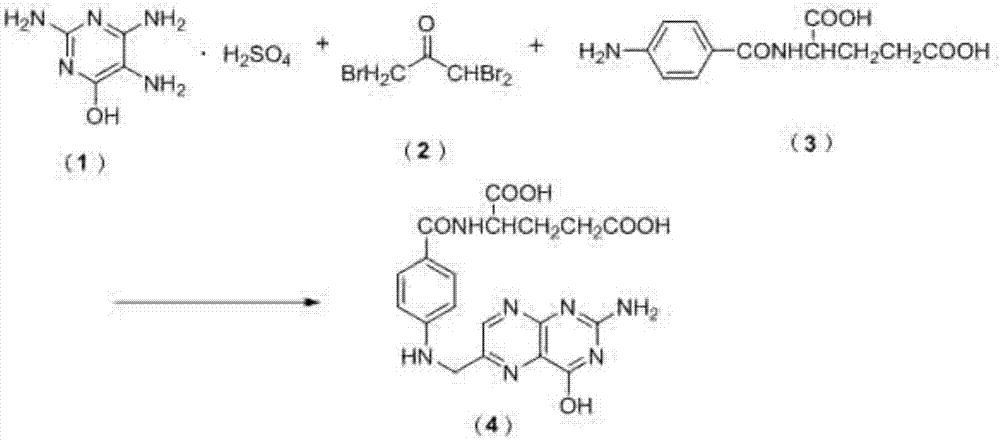
[0026] A preparation method for folic acid, specifically comprising the following steps:
[0027] (1) Add 18g of 1,1,3-tribromoacetone into 600mL of ethanol solution, stir and heat to 55°C;
[0028] (2) After the 1,1,3-tribromoacetone is completely dissolved, continue to add 10 g of p-aminobenzoyl-L-glutamic acid and stir until completely dissolved to obtain the first reaction solution;
[0029] (3) Add 120 g of water to another reaction flask, weigh 12 g of 2,4,5-triamino-6-hydroxypyrimidine sulfate in batches, and adjust the pH to 8 with saturated sodium carbonate to 2 , 4,5-triamino-6-hydroxypyrimidine sulfate is completely dissolved to obtain the second reaction solution;
[0030] (4) The second reaction solution is added in batches to the first reaction solution, and the insulation reaction is carried out for 5 hours;
[0031] (5) After the reaction is completed, carry out suction filtration to obtain the folic acid crude product;
[0032] (6) Add the folic acid crude ...
Embodiment 2
[0043] A preparation method for folic acid, specifically comprising the following steps:
[0044] (1) Add 14.74g of 1,1,3-tribromoacetone into 600mL of ethanol solution, stir and heat to 50°C;
[0045] (2) After the 1,1,3-tribromoacetone is completely dissolved, continue to add 10.65 g of p-aminobenzoyl-L-glutamic acid and stir until completely dissolved to obtain the first reaction solution;
[0046] (3) Add 90 g of water to another reaction flask, weigh 9.49 g of 2,4,5-triamino-6-hydroxypyrimidine sulfate in batches, and adjust the pH to 8.5 with saturated sodium carbonate until 2,4,5-triamino-6-hydroxypyrimidine sulfate is completely dissolved to obtain the second reaction solution;
[0047] (4) The second reaction solution is added in batches to the first reaction solution, and the insulation reaction is carried out for 6 hours;
[0048] (5) After the reaction is completed, carry out suction filtration to obtain the folic acid crude product;
[0049] (6) Add the folic a...
Embodiment 3
[0057] A preparation method for folic acid, specifically comprising the following steps:
[0058] (1) Add 20.63g of 1,1,3-tribromoacetone into 600mL of ethanol solution, stir and heat to 40°C;
[0059] (2) After the 1,1,3-tribromoacetone is completely dissolved, continue to add 5.33 g of p-aminobenzoyl-L-glutamic acid and stir until completely dissolved to obtain the first reaction solution;
[0060] (3) Add 144g of water in another reaction flask, weigh 14.23g of 2,4,5-triamino-6-hydroxypyrimidine sulfate in batches, and adjust the pH to 7.5 with saturated sodium carbonate until 2,4,5-triamino-6-hydroxypyrimidine sulfate is completely dissolved to obtain the second reaction solution;
[0061] (4) adding the second reaction solution in batches to the first reaction solution, and performing an insulation reaction for 8 hours;
[0062] (5) After the reaction is completed, carry out suction filtration to obtain the folic acid crude product;
[0063] (6) Add the folic acid crude ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com