Method for inducing directional myocardiac differentiation of iPSCs (induced Pluripotent Stem Cells) with H9C2 myocardial cell culture solution
A technique for culturing cardiomyocytes and cells, which is applied in the field of medicine and can solve various problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Preparation of iPSCs
[0020] 1) Preparation of the trophoblast layer (MEF): 0.1% (volume fraction) of gelatin is added to the T25 culture flask, placed in a 37°C cell incubator for 20 minutes, and then sucked out, and 5-6mL preheated 37°C MEF culture medium is added At the same time, the mouse embryonic fibroblasts (MEF) (purchased from the cell bank of the Shanghai Chinese Academy of Sciences) were quickly taken out of liquid nitrogen, placed in a 37°C water bath and quickly thawed and immediately wiped and stored with 75% alcohol with a volume fraction of alcohol. After the tube is taken to the ultra-clean table, transfer the cell suspension in the cryopreservation tube to a 15 mL centrifuge tube containing MEF medium, centrifuge at 1000 rpm for 5 minutes, discard the supernatant and resuspend it and add it to a T25 culture flask, and place it in CO 2 In a constant temperature incubator, iPSCs can be added to the feeder layer after 24 hours of cultivation;
[00...
Embodiment 2H9
[0022] Example 2H9C2 cardiomyocyte culture medium induces differentiation of iPSCs
[0023] H9C2 cardiomyocytes were resuspended in DMEM medium containing 15% FBS, 50U / mL penicillin and 10μg / mL streptomycin, and then inoculated in a T25 culture flask at an appropriate cell density for culture, and passaged for 1 to 2 days. Aspirate the culture solution before subculture for 3 days and filter it through a 0.22 μm filter membrane, then the induction culture solution, that is, the H9C2 cardiomyocyte culture solution, is ready for use.
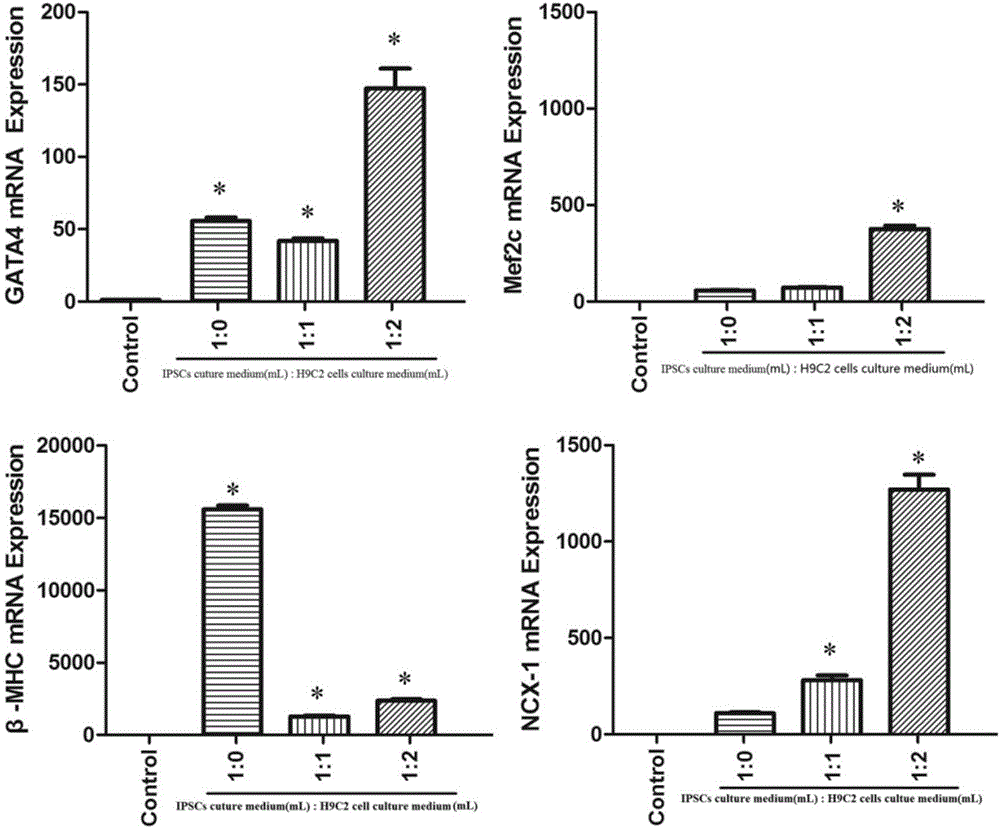
[0024] Take the iPSCs in the logarithmic growth phase, and take the 6 After cells / mL iPSCs were cultured in hanging drop for 48 hours, they were inoculated in a six-well plate pretreated with 0.1% gelatin for 1 hour, and the pre-prepared conventional iPSCs cell culture medium without LIF and H9C2 cardiomyocyte culture medium were adjusted according to 1:0, 1:1 , 1:2 ratio mixed to prepare 15% fetal bovine serum differentiation medium to culture iPSCs. ...
Embodiment 3
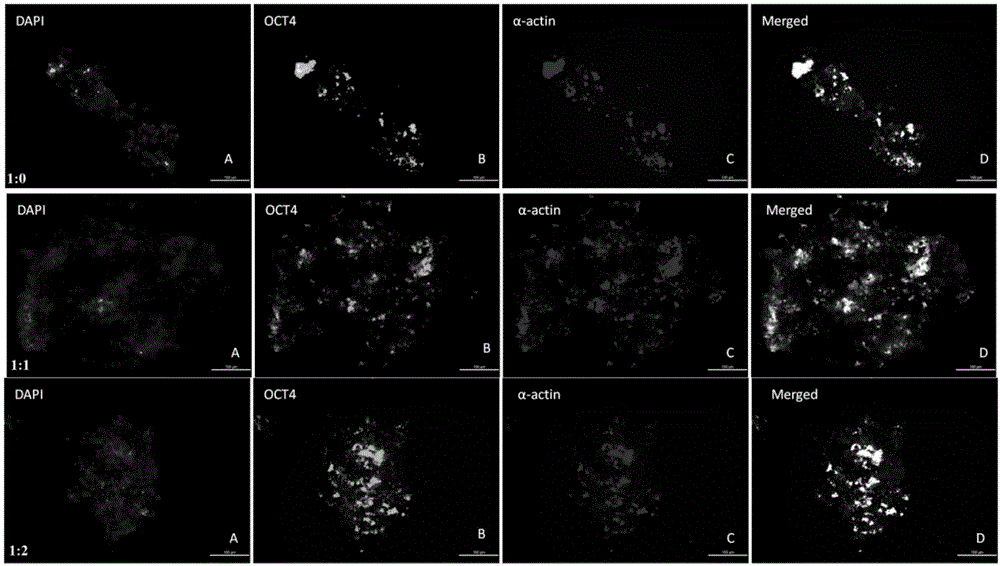
[0026] Example 3 Inverted microscope and cell immunofluorescence detection of multidirectional differentiation potential genes Oct-4-EGFP and α-sarcomeric actin (α-sarcomeric actin, α-actin) expression
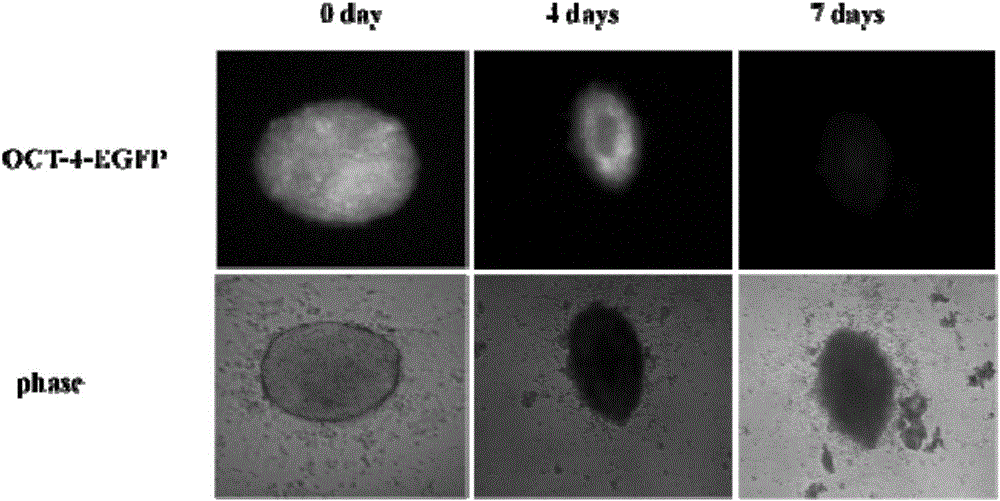
[0027] After induction and culture for 14 days, wash 3 times with PBS, fix with 4% paraformaldehyde, wash with PBS, block with 5% BSA, add rabbit anti-mouse monoclonal primary antibody α-actin (1:500), overnight at 4 degrees, PBS Rinse, drop PE-labeled goat anti-rabbit IgG (1:50), incubate at 37°C in the dark for 30 minutes, rinse the nucleus with DAPI for 10 minutes after rinsing with PBS, and mount and fix with resin glue. Observe under a fluorescence microscope, with 10 fields of view in each hole, a total of 30 fields of view.
[0028] Changes of EGFP expression in induced pluripotent stem cells, such as figure 1 As shown, the results show that: iPSCs cell culture medium and H9C2 cardiomyocyte culture medium induce their differentiation at a volume ratio of 1:2. It can be seen ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com