Novel prothetic group connecting arm for synthesizing diubiquitin and synthesis method of diubiquitin
A synthetic method and linker technology, applied in the field of ubiquitin, can solve the problems of low coupling reaction efficiency and achieve high-efficiency reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the synthesis of prosthetic group connecting arm
[0038] The reaction formula is as follows:
[0039]
[0040] The specific steps are:
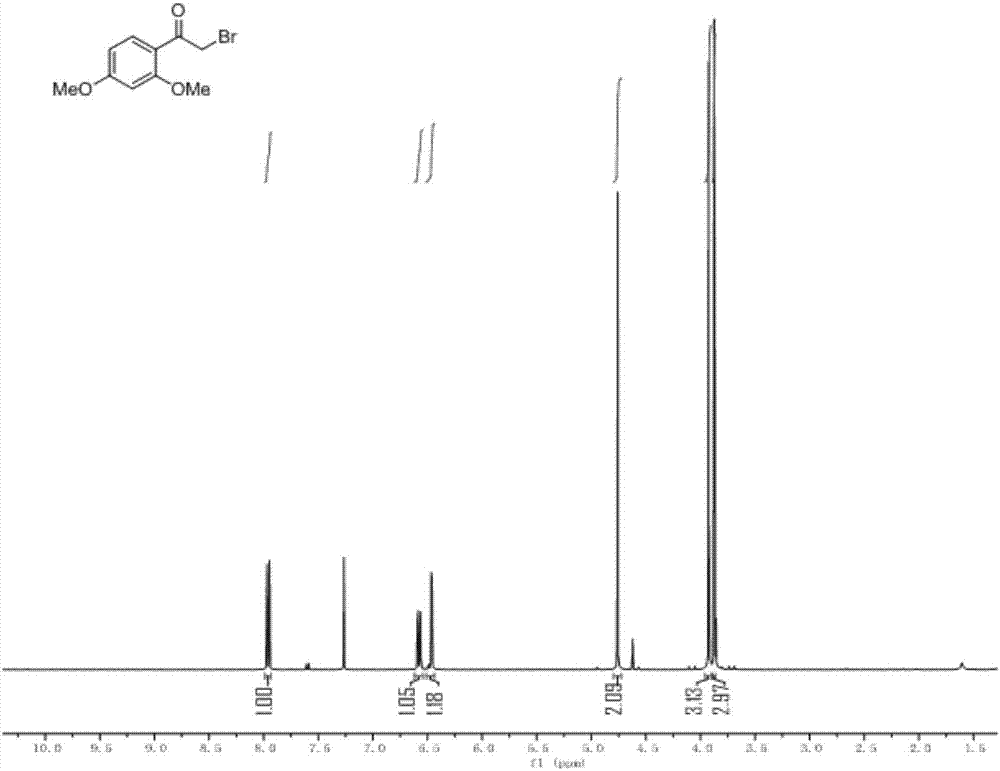
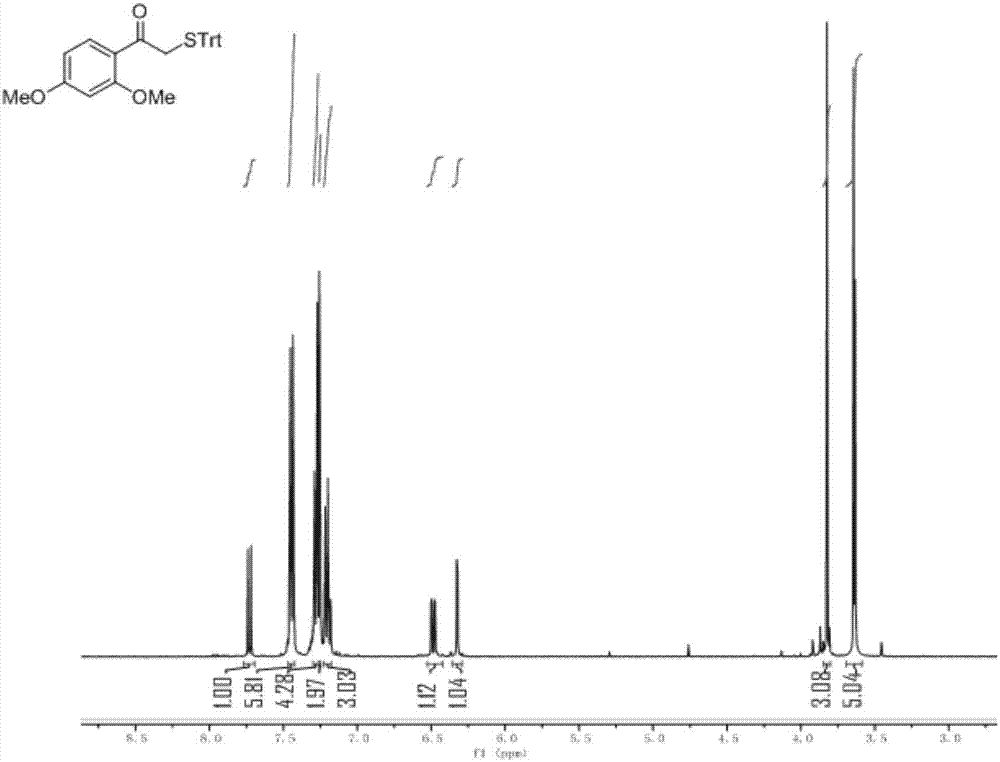
[0041] Compound II: Take 1.0g of Compound I (3.86mmol) and 1.28g of TrtSH (4.63mmol) in a 100ml round-bottomed flask equipped with a magnet, add 25ml of N, N-dimethylformamide (DMF) to dissolve, slowly add 808ulN , N-diisopropylethylamine (DIEA, 4.64mmol), stirred and reacted at room temperature, reacted for 2h, added a large amount of water to the flask for dilution, extracted 3 times with ethyl acetate (EA), and washed 3 times with water, The organic phase was collected and dried over anhydrous sodium sulfate. The dried organic phase was filtered, and the solvent was spin-dried by a rotary evaporator to obtain 1.55 g of the product as a white solid with a yield of 88.35%.
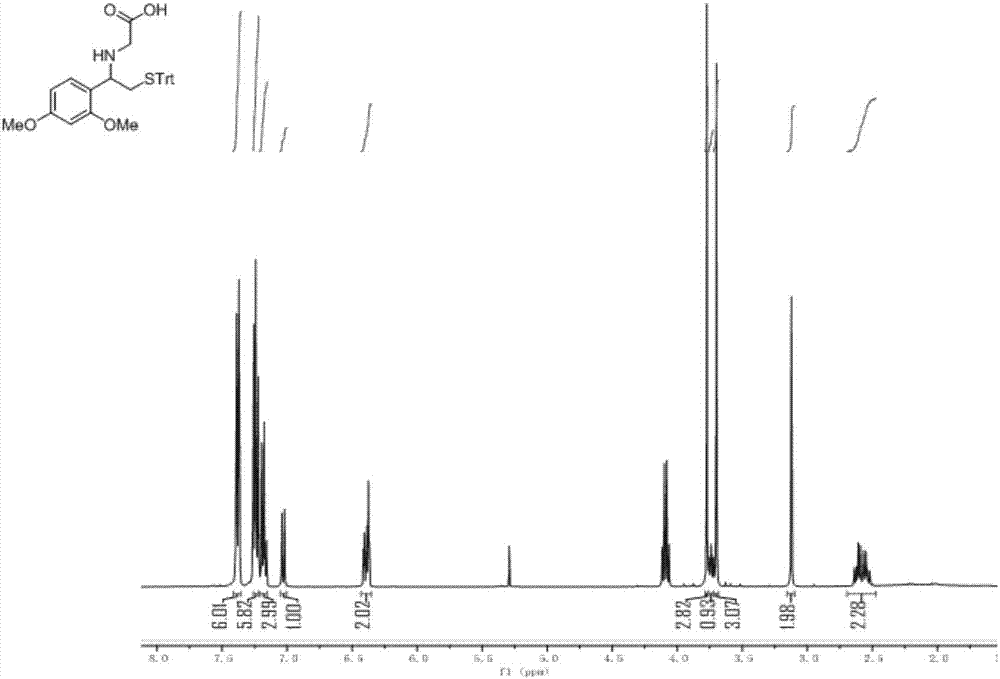
[0042] Compound III: Take 0.9g of Compound II (2.0mmol) in a 150ml round bottom flask equipped with a magnet, add 15ml of anhydrous methanol t...
Embodiment 2
[0045]Example 2: Synthesis of diubiquitin K48C
[0046] The reaction formula is as follows:
[0047]
[0048] The specific steps are:
[0049] Load small molecules: In a 50ml reaction tube equipped with magnetons, add protein Ub(1-76)K48C (3umol, 26mg), prosthetic linker (240ul of 500mmol stock solution), initiator 2,2-dihydroxy Methylbutyric acid (DMPA, 150ul of 200mmol stock solution), tert-butyl mercaptan (27ul), was supplemented to 3ml with a mixed solvent (water:N-methylpyrrolidone=1:1). The reaction was stirred at 365nm light wavelength for 2h, dialyzed overnight, separated on a semi-preparative column, and the isolated product was lyophilized to obtain 18 mg, with a yield of 67.7%.
[0050] Deprotection: In a 50ml reaction tube equipped with a magneton, add 18mg (2umol) of ubiquitin equipped with a prosthetic linking arm in the above step, and use 2ml of buffer (6M guanidine hydrochloride, 0.2MNaH 2 PO 4 , 0.2M methoxyamine, PH4) dissolved, stirred and reacted at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com