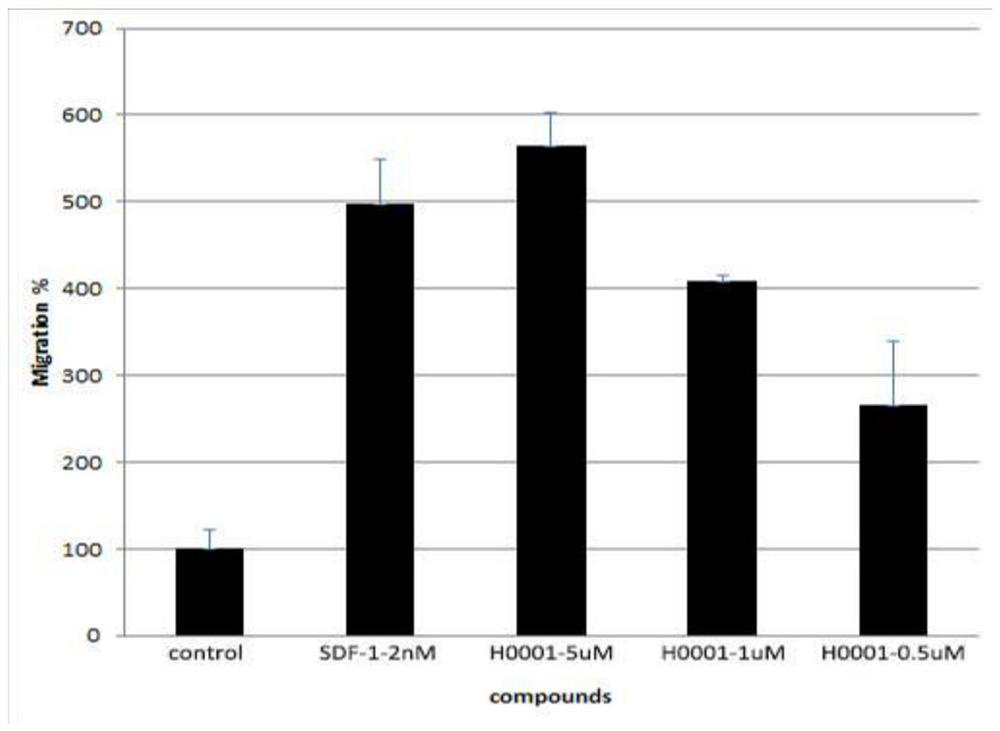
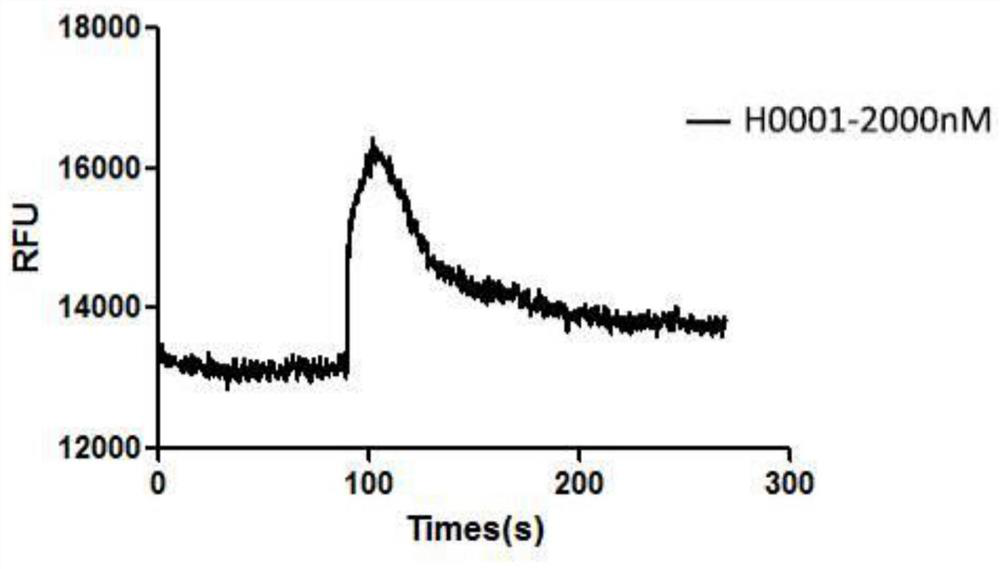
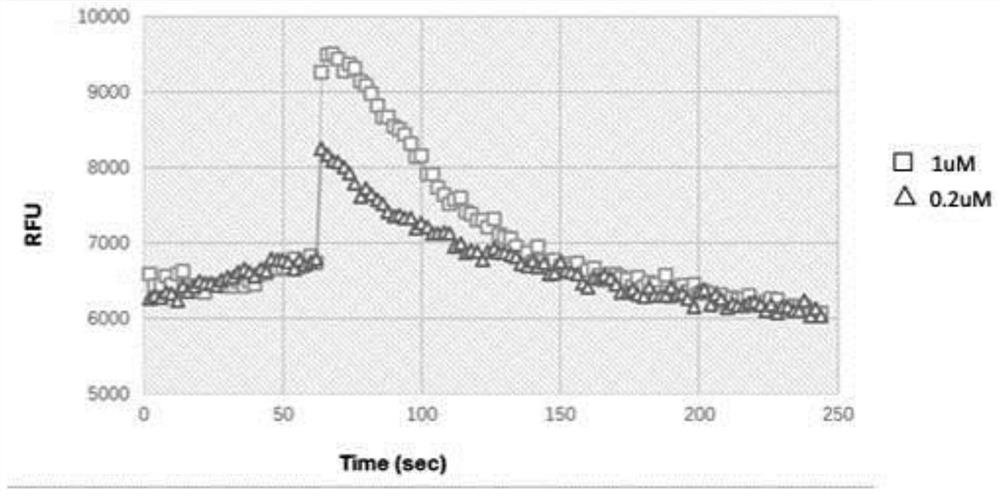
A polypeptide having cxcr4 protein agonistic activity and its application and pharmaceutical composition
A composition and drug technology, applied in the field of polypeptides with CXCR4 protein agonistic activity and their applications and pharmaceutical compositions, can solve the problems of lack of treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: Synthesis of H0001
[0083] Adopt Fmoc solid-phase synthesis method to synthesize, and concrete preparation method is as follows:
[0084] Weigh 357mg of Rink Amide-MBHA resin with a substitution degree of 0.28mmol / g in a solid phase reactor, add DCM to swell for 30min, then add 20% PIP / DMF solution for deprotection twice, the first time is 5min, and the second time is 20min . Wash several times with DCM and DMF respectively, and drain. It was detected by Kaiser test, and the resin or solution showed blue color, indicating that it had been deprotected.
[0085] Add Fmoc-D-Pro-OH (169mg, 0.5mmol), DIC (103mg, 0.5mmol), HOBt (68mg, 0.5mmol), DMF (4mL) into the solid phase reactor, and react at room temperature for 2h. After the coupling is completed, wash with DCM and DMF several times, and drain. Then use the Kaiser test to detect, and proceed to the next step after the detection is passed. Wash and dry the resin to obtain Fmoc-Pro-Rink Amide-MBHA resin. ...
Embodiment 2
[0088] The synthesis of embodiment 2 H0006
[0089] Weigh 357mg of Rink Amide-MBHA resin with a substitution degree of 0.28mmol / g in a solid phase reactor, add DCM to swell for 30min, then add 20% PIP / DMF solution for deprotection twice, the first time is 5min, and the second time is 20min . Wash several times with DCM and DMF respectively, and drain. It was detected by Kaiser test, and the resin or solution showed blue color, indicating that it had been deprotected.
[0090]Add Fmoc-Lys(Dde)-OH (266mg, 0.5mmol), DIC (103mg, 0.5mmol), HOBt (68mg, 0.5mmol), DMF (4mL) into the solid phase reactor, and react at room temperature for 2h. After the coupling is completed, the resin is washed and dried to obtain Fmoc-Lys(Dde)-Rink Amide-MBHA resin. The Kaiser test is used for detection, and the next reaction is carried out after the detection is passed. Add 20% PIP / DMF solution for deprotection twice, the first time is 5 minutes, the second time is 20 minutes, and the Fmoc protect...
Embodiment 3
[0095] Embodiment 3: polypeptide compound of the present invention
[0096] The amino acid sequence of the CXCR4 activity regulating polypeptide is shown in Table 1, and was synthesized according to the method described in Example 1 or Example 2.
[0097] Table 1 CXCR4 activity regulating polypeptide and its amino acid sequence
[0098]
[0099] a Italics represent D-type amino acids
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com