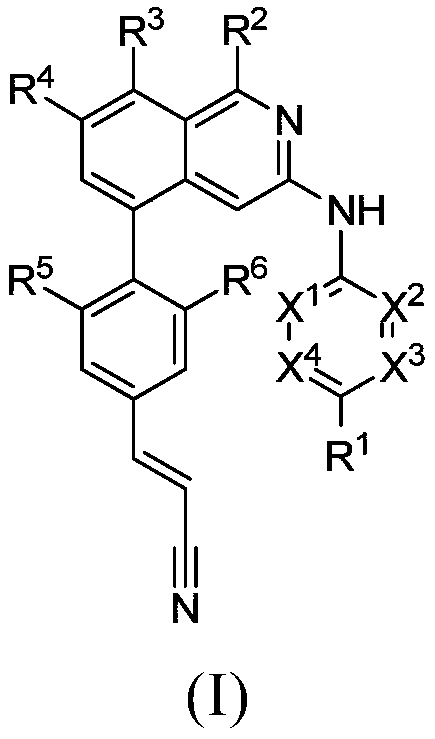
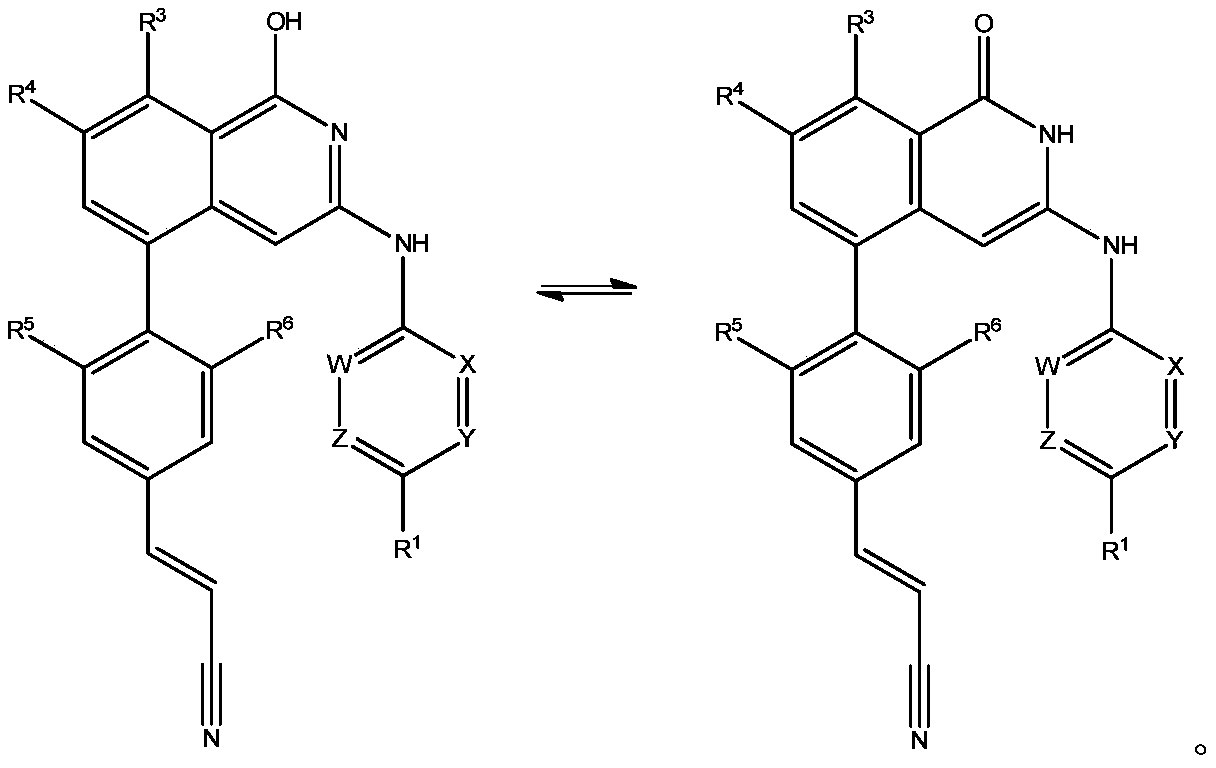
Isoquinoline compounds for hiv treatment
A kind of technology of compound and therapeutic agent, applied in the field of isoquinoline compound used for HIV treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0372] (E)-2-((1-amino-5-(4-(2-cyanovinyl)-2,6-dimethylphenyl)isoquinolin-3-yl)amino)pyrimidine-5- Formonitrile-Compound 1
[0373]
[0374] Step 1: Synthesis of 4-bromo-2-(hydroxyimino)-2,3-dihydro-1H-inden-1-one (compound 1a)
[0375]
[0376] 4-Bromo-2,3-dihydro-1H-inden-1-one (10g, 47.4mmol, Ark Pharm, Inc.--AK-31085) was dissolved in ether (120mL), ethanol (50mL) and dichloromethane ( 20mL) in the mixture. The reaction mixture was cooled to 0°C and 1M HCl (24 mL, 24 mmol) in ether was added, and then a 15% ethyl nitrite solution (60 mL, 104 mmol) in ethanol was slowly added to the reaction mixture at 0°C over 1 hour. The reaction mixture was vigorously stirred at room temperature for 3 hours and then concentrated under reduced pressure. Ether (50 mL) was added and the mixture was cooled to 0°C. The solid product was filtered and washed twice with ether (2×20 mL) to produce compound 1a. 1 H NMR(400MHz, DMSO-d 6 )δ12.83(s,1H),7.98(d,J=7.8Hz,1H), 7.77(d,J=7.6Hz,1H), 7.62-7.22...
Embodiment 2
[0396] (E)-5-((1-Amino-5-(4-(2-cyanovinyl)-2,6-dimethylphenyl)isoquinolin-3-yl)amino)pyrazine-2 -Formonitrile-Compound 2
[0397]
[0398] (E)-5-((1-Amino-5-(4-(2-cyanovinyl)-2,6-dimethylphenyl)isoquinolin-3-yl)amino)pyrazine-2 -Synthesis of Carbonitrile (Compound 2)
[0399]
[0400] Compound 1f (200mg, 0.60mmol), 5-aminopyrazine-2-carbonitrile (163mg, 1.36mmol, Ark PharmInc, AK-21935), N,N-diisopropylethylamine (835μL, 4.78mmol), (9,9-Dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphine) (35mg, 0.06mmol) and palladium(II) acetate (13mg, 0.06mmol) in argon The following are combined in N-methyl-2-pyrrolidone (1 mL). The reaction was heated in a sealed container at 120°C for 4 hours. The reaction mixture was cooled to room temperature and purified by reverse phase chromatography (20-60% aqueous acetonitrile with 0.1% trifluoroacetic acid) to provide the TFA salt of compound 2. 1 HNMR (400MHz, methanol-d 4 )δ8.55(d,J=1.4Hz,1H), 8.36(dd,J=8.1,1.4Hz,1H), 8.31(d,J=1.4Hz,1H),7.73-7.5...
Embodiment 3
[0402] (E)-6-((1-amino-5-(4-(2-cyanovinyl)-2,6-dimethylphenyl)isoquinolin-3-yl)amino)pyridazine-3 -Formonitrile-Compound 3
[0403]
[0404] (E)-6-((1-amino-5-(4-(2-cyanovinyl)-2,6-dimethylphenyl)isoquinolin-3-yl)amino)pyridazine-3 -Synthesis of Carbonitrile (Compound 3)
[0405]
[0406] Compound 1f (200mg, 0.60mmol), 6-aminopyridazine-3-carbonitrile (163mg, 1.36mmol, MatrixScientific, 112287), N,N-diisopropylethylamine (835μL, 4.78mmol), (9, 9-Dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphine) (35mg, 0.06mmol) and palladium(II) acetate (13mg, 0.06mmol) were combined under argon N-methyl-2-pyrrolidone (1 mL). The reaction was heated in a sealed container at 120°C for 4 hours. The reaction mixture was cooled to room temperature and purified by reverse phase chromatography (20-60% aqueous acetonitrile with 0.1% trifluoroacetic acid) to provide the TFA salt of compound 3. 1 H NMR (400MHz, methanol-d 4 )δ8.45-8.31(m,1H),7.94(d,J=9.3Hz,1H),7.81-7.43(m,5H),7.24(d,J=9.3Hz,1H),6.30(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com