Preparation method of triple inactivated vaccine for swine erysipelas, porcine parvovirus and swine influenza virus
A swine flu virus and parvovirus technology, applied in the field of vaccines, can solve problems such as the difficulty of inoculating multiple pathogens at one time, and achieve the effect of improving immunity and production performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A preparation method of porcine erysipelas, porcine parvovirus and swine influenza virus triple inactivated vaccine, the steps are as follows:
[0035] 1 Production Seed Preparation
[0036] 1.1 Erysipelas suis production seed preparation
[0037] 1.1.1 Primary seed propagation
[0038] After unsealing the Erysipelas suis freeze-dried strains, add Martin broth to dilute and streak inoculate on the blood agar slope, culture at 37°C for 24 hours, streak on 10% serum Martin agar plate, culture for 24-36 hours, select a typical uniform Several bacterial colonies were inoculated on the slant of blood agar, cultured at 37°C for 24 hours, and used as primary seeds.
[0039] 1.1.2 Secondary seed propagation
[0040] Take the first-grade seeds and inoculate them in Martin Broth, incubate them at 37°C for 24 hours, then inoculate them in a large amount of meat liver and stomach digestion soup or Martin Broth, and incubate them for 24 hours, conduct a pure inspection according ...
Embodiment 3
[0083] Example 3 Vaccine Efficacy Research
[0084] 1 Experimental design
[0085] 1.1 Vaccine Immunization
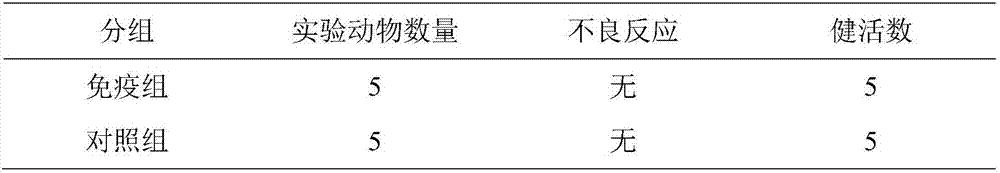
[0086] Get the vaccine prepared in Example 1, mark the portion by the bottle label, 2 mL per head, intramuscularly inject 1 portion into 15 healthy susceptible pigs with a body weight of more than 20 kg 1-2 months after weaning, and 21 days after the first immunization. The dose and method of immunization were the same, and a control group of 15 animals was set up without immunization.
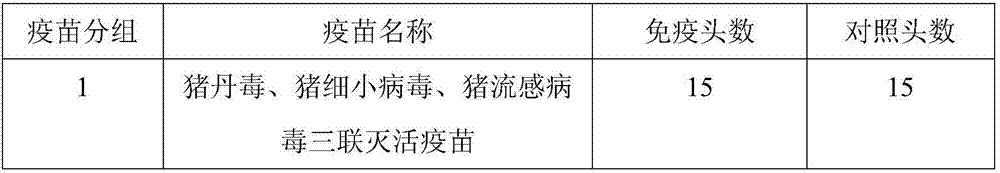
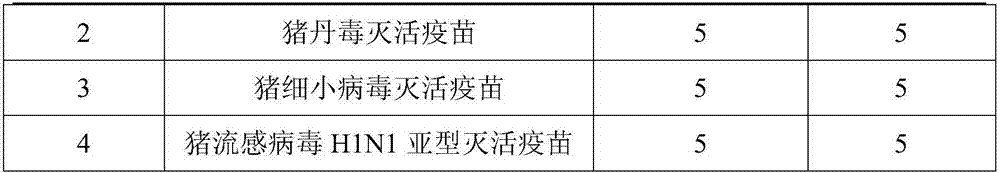
[0087]Purchase commercially available porcine erysipelas inactivated vaccine (C43-5), porcine parvovirus inactivated vaccine (CP-99), swine influenza virus H1N1 subtype inactivated vaccine, and inject the post-weaning 1 - 5 healthy susceptible pigs weighing more than 20kg for 2 months. The vaccine groups are shown in the table below.
[0088] Table 2 Vaccine immunization groups
[0089]
[0090]
[0091] 1.2 Antivirus
[0092] Three weeks after immunization, erysipelas, por...
Embodiment 4
[0101] The preparation method of porcine erysipelas, porcine parvovirus and swine influenza virus triple inactivated vaccine comprises the following steps:
[0102] 1) Cultivate the Erysipelas suis type 2 C43-5 strain to prepare a live bacterial liquid;
[0103] 2) Adjust the antigen concentration in the live Erysipelas suis type 2 C43-5 liquid obtained in step 1) to 10 by diluting or centrifuging. 9 CFU / mL;
[0104] 3) Utilizing formaldehyde to inactivate the Erysipelas suis type 2 C43-5 bacterium liquid after step 2) adjusting the concentration;
[0105] 4) Breeding porcine parvovirus BJ-2 strain to prepare live venom;
[0106] 5) using formaldehyde to inactivate the porcine parvovirus BJ-2 strain venom in step 4);
[0107] 6) Propagate swine influenza virus H1N1 strain to prepare live venom;
[0108] 7) using formaldehyde to inactivate the swine influenza virus H1N1 strain venom in step 6);
[0109] 8) The Erysipelas suis C43-5 bacterium liquid after step 3) inactivati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com