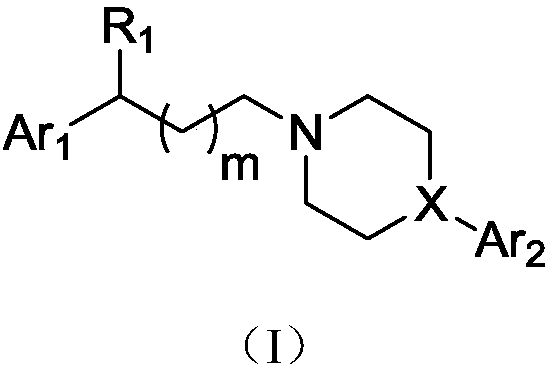
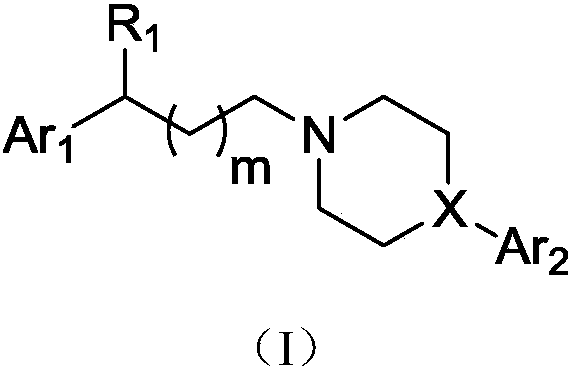
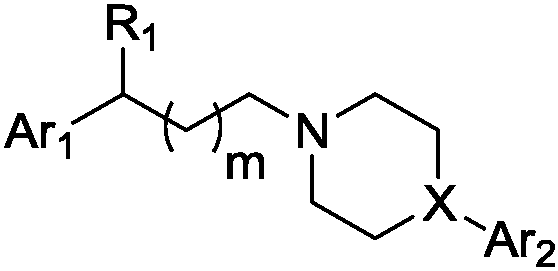
Aralkyl heterocyclic derivative and application of aralkyl heterocyclic derivative in multi-target depression resistance
A technology of heterocycles and aranes, applied in the field of new drug design and synthesis, can solve problems such as low curative effect, large side effects, and inability to improve cognitive impairment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] 3-(4-(1,2-Benzisothiazol-3-yl)-1-piperazin)yl-1-(3,4-dichlorophenyl)yl-propanol (IV-1) hydrochloride preparation of
[0135] 3-Chloro-1-(3,4-dichlorophenyl)-acetone (0.05mol), piperazine compound hydrochloride (0.05mol), diisopropylethylamine (0.2mol) dissolved in acetonitrile (100mL) According to the operation of General Method 1, 14.71 g of the intermediate (white solid) was obtained, and the yield was 70%. MS(m / z): 420.1[M+1] + .
[0136] The above intermediate (10mmol), sodium borohydride (10mmol) in methanol (50mL), according to general method two operations, prepared 3-(4-(1,2-benzisothiazol-3-yl)-1- Piperazin)-1-(3,4-dichlorophenyl)-propanol hydrochloride (white solid) 4.13g, yield 90%. MS(m / z): 422.1[M+1] + .
[0137] 1 H NMR (400MHz, DMSO) δ10.85 (s, 1H), 8.12 (t, J = 7.7Hz, 2H), 7.64 (dd, J = 5.1, 3.2Hz, 2H), 7.60 (dd, J = 11.6, 4.4Hz, 1H), 7.47(dd, J=11.6, 4.4Hz, 1H), 7.39(dd, J=8.4, 1.9Hz, 1H), 5.84(s, 1H), 4.75(d, J=5.0Hz, 1H), 4.05(d, J=13.2Hz, 2H...
Embodiment 2
[0139] 3-(4-(6-fluoro-1,2-benzisothiazol-3-yl)-1-piperidinyl-1-(3,4-dichlorophenyl)yl-propanol (IV-2 ) preparation of hydrochloride
[0140] 3-Chloro-1-(3,4-dichlorophenyl)-acetone (0.05mol), piperidine compound hydrochloride (0.05mol), diisopropylethylamine (0.2mol) dissolved in acetonitrile (100mL) According to the operation of General Method 1, 13.06 g of the intermediate (white solid) was obtained, and the yield was 62%. MS(m / z): 421.1[M+1] + .
[0141] The above intermediate (10mmol), sodium borohydride (10mmol) in methanol (50mL), according to general method two operations, prepared 3-(4-(6-fluoro-1,2-benzisothiazol-3-yl )-1-piperidinyl-1-(3,4-dichlorophenyl)yl-propanol hydrochloride (white solid) 4.14g, yield 90%. MS(m / z): 423.2[M+1] + .
[0142] 1 H NMR (400MHz, DMSO) δ10.85(s, 1H), 8.00(dd, J=8.8, 5.3Hz, 1H), 7.69(dd, J=9.1, 2.1Hz, 1H), 7.59(t, J= 5.1Hz, 2H), 7.35(dd, J=8.3, 1.9Hz, 1H), 7.28(td, J=9.2, 2.2Hz, 1H), 5.70(s, 1H), 4.68(t, J=6.2Hz, 1H), 3.14(ddd, ...
Embodiment 3
[0144] 3-(4-([1,1'-biphenyl]-2-yl)-1-piperazinyl-1-(3,4-dichlorophenyl)yl-propanol (IV-3) salt Salt preparation
[0145] 3-Chloro-1-(3,4-dichlorophenyl)-acetone (0.05mol), piperazine compound hydrochloride (0.05mol), diisopropylethylamine (0.2mol) dissolved in acetonitrile (100mL) According to the operation of General Method 1, 13.18 g of the intermediate (white solid) was obtained, and the yield was 60%. MS(m / z): 439.2[M+1] + .
[0146] The above intermediate (10mmol), sodium borohydride (10mmol) in methanol (50mL), according to general method two operations, prepared 3-(4-([1,1'-biphenyl]-2-yl)- 4.54 g of 1-piperazinyl-1-(3,4-dichlorophenyl)-propanol hydrochloride (white solid), yield 95%. MS(m / z): 441.2[M+1] + .
[0147] 1H NMR (400MHz, DMSO) δ10.85(s, 1H), 7.67–7.57(m, 4H), 7.45(t, J=7.6Hz, 2H), 7.37–7.30(m, 3H), 7.24(dd, J=7.6,1.6Hz,1H),7.17–7.08(m,2H),4.68(s,1H),3.40(d,J=11.4Hz,2H),3.17–3.04(m,4H),3.00–2.82 (m,4H),2.09–1.91(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com