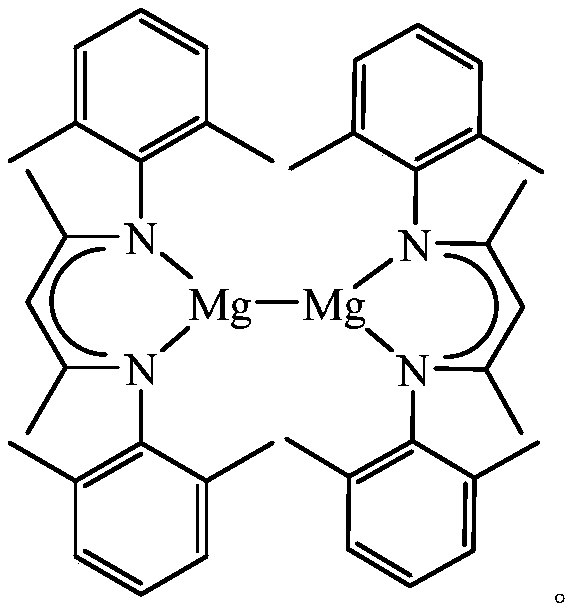
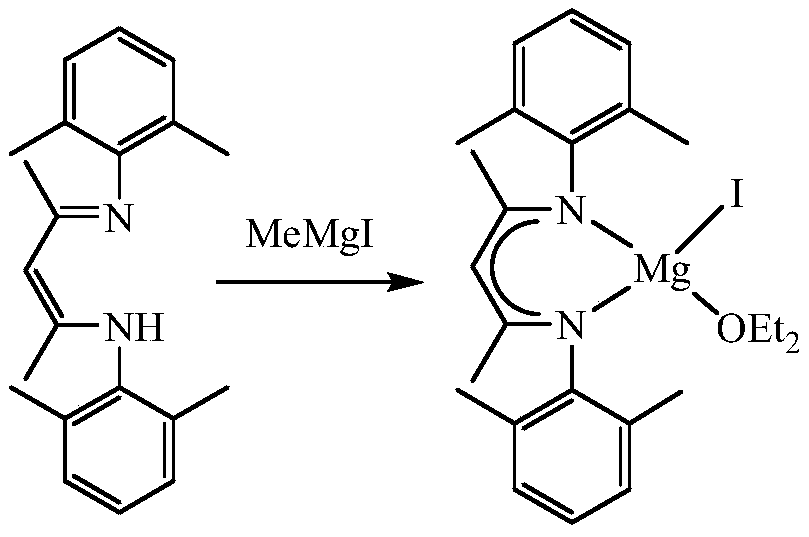
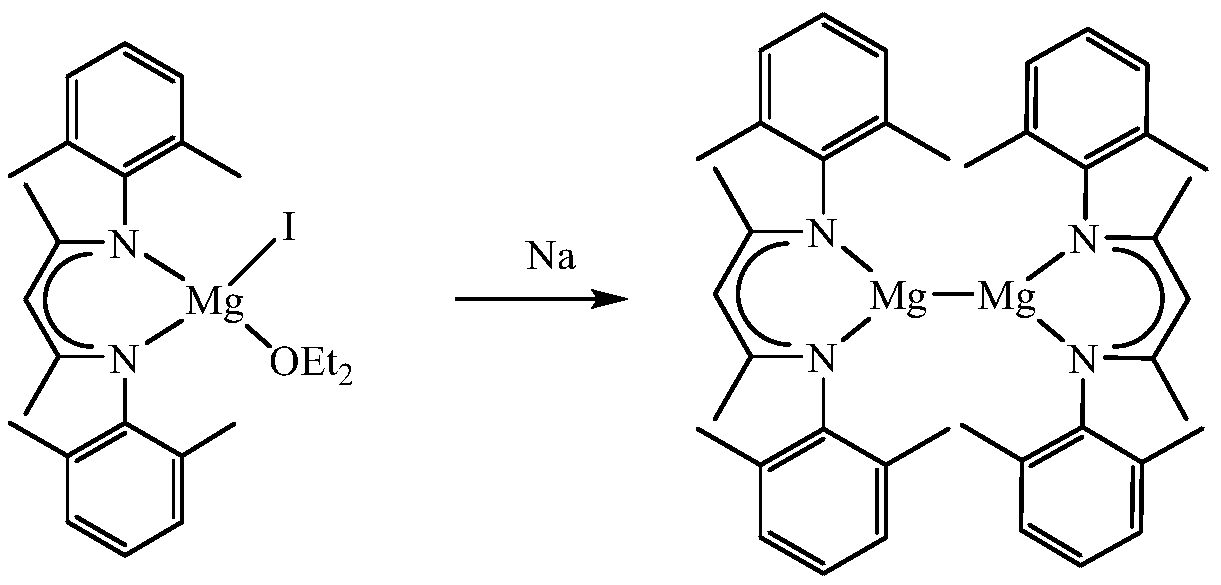
A kind of β-diimine monovalent magnesium compound and its preparation method and application in hydroboration of aldehydes and ketones
A technology of magnesium compound and diimine, applied in the field of metal organic compound preparation, can solve the problem that the application of the catalytic amount of monovalent magnesium compound is not reported, and achieve the effects of simple and easy operation of the reaction process, easy product and low toxicity of the article.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of the iodide of β-diimine magnesium, the process is as follows:
[0025] In the absence of water and oxygen, 3.27 mmol of β-diimine ligand was dissolved in 25 mL of ether solution in a single-port reaction tube, and 3.92 mmol of methylmagnesium iodide was added dropwise to the above solution at -80°C, and reacted at room temperature for 24 hours. After filtration, the solid was sucked dry, and the filtrate was concentrated to 5 mL to obtain colorless crystals. The mass of solid and crystals was 1.63 g, and the yield was 94%. M.p. 271-273°C. NMR spectrum: 1 HNMR (600MHz, C 6 D. 6 ):δ6.99-6.91(m,6H,Ar-H),4.88(s,1H,CH),3.12(s,4H,OCH 2 CH 3 ),2.65(s,6H,CH 3 ),2.08(s,6H,CH 3 ),1.55(s,6H,NCCH 3 ),0.48(s,6H,OCH 2 CH 3 ) ppm. 13 C{ 1 H}NMR (151MHz, C 6 D. 6 ):δ168.87 (NCCH 3 ), 147.75, 131.57, 129.56, 124.76 (Ar-C), 95.31 (=CH), 65.96 (OCH 2 CH 3 ),23.52(OCH 2 CH 3 ), 21.09 (NCCH 3 ), 18.89, 13.15 (CH 3 ) ppm.
Embodiment 2
[0027] The preparation of the iodide of β-diimine magnesium, the process is as follows:
[0028] In the absence of water and oxygen, 3.27 mmol of β-diimine ligand was dissolved in 25 mL of ether solution in a single-port reaction tube, and 3.60 mmol of methylmagnesium iodide was added dropwise to the above solution at -60°C, and reacted at room temperature for 15 h. After filtration, the solid was sucked dry, and the filtrate was concentrated to 5 mL to obtain colorless crystals. The mass of solid and crystals was 1.61 g, and the yield was 92%. M.p. 271-273°C. NMR spectrum: 1 HNMR (600MHz, C 6 D. 6 ):δ6.99-6.91(m,6H,Ar-H),4.88(s,1H,CH),3.12(s,4H,OCH 2 CH 3 ),2.65(s,6H,CH 3 ),2.08(s,6H,CH 3),1.55(s,6H,NCCH 3 ),0.48(s,6H,OCH 2 CH 3 ) ppm. 13 C{ 1 H}NMR (151MHz, C 6 D. 6 ):δ168.87 (NCCH 3 ), 147.75, 131.57, 129.56, 124.76 (Ar-C), 95.31 (=CH), 65.96 (OCH 2 CH 3 ),23.52(OCH 2 CH 3 ), 21.09 (NCCH 3 ), 18.89, 13.15 (CH 3 ) ppm.
Embodiment 3
[0030] The preparation of the iodide of β-diimine magnesium, the process is as follows:
[0031] In the absence of water and oxygen, 3.27 mmol of β-diimine ligand was dissolved in 25 mL of ether solution in a single-port reaction tube, and 3.27 mmol of methylmagnesium iodide was added dropwise to the above solution at -40°C, and reacted at room temperature for 15 h. After filtration, the solid was sucked dry, and the filtrate was concentrated to 5 mL to obtain colorless crystals. The mass of solid and crystals was 1.63 g, and the yield was 94%. M.p. 271-273°C. NMR spectrum: 1 HNMR (600MHz, C 6 D. 6 ):δ6.99-6.91(m,6H,Ar-H),4.88(s,1H,CH),3.12(s,4H,OCH 2 CH 3 ),2.65(s,6H,CH 3 ),2.08(s,6H,CH 3 ),1.55(s,6H,NCCH 3 ),0.48(s,6H,OCH 2 CH 3 ) ppm. 13 C{ 1 H}NMR (151MHz,C 6 D. 6 ):δ168.87 (NCCH 3 ), 147.75, 131.57, 129.56, 124.76 (Ar-C), 95.31 (=CH), 65.96 (OCH 2 CH 3 ),23.52(OCH 2 CH 3 ), 21.09 (NCCH 3 ), 18.89, 13.15 (CH 3 ) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com