Method of preparing DNA probe, kit and application thereof
A technology of DNA probes and biological products, applied in the field of biomedicine, can solve the problems of different fragment sizes, many interference factors, and difficulties, and achieve the effects of high safety, high specificity, and improved specificity and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
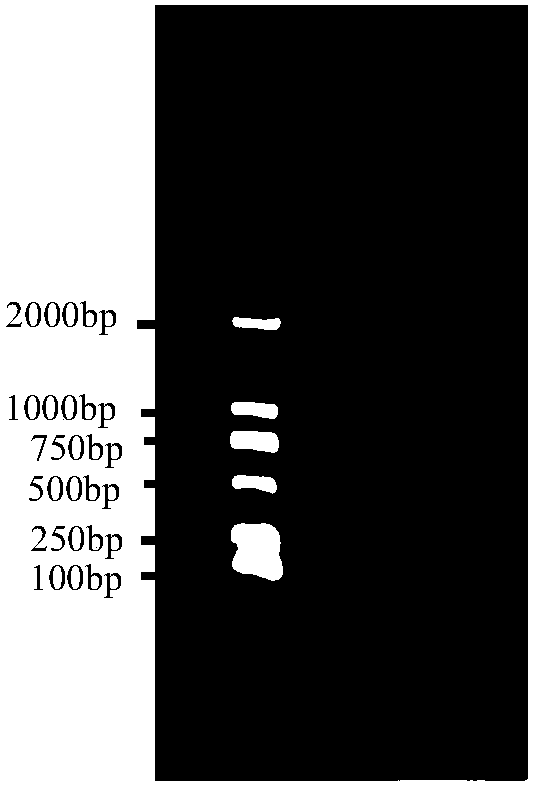
[0042] The preparation of embodiment 1 probe DNA
[0043] The inventor explored a variety of experimental conditions when preparing the probe DNA in the early stage, so as to break the Escherichia coli genomic DNA into fragments of appropriate size, so that the fragment size is suitable for DNA hybridization and probe labeling. Specifically, the inventors screened a large number of conditions for ultrasonic treatment of Escherichia coli genomic DNA, and finally screened out test conditions suitable for the preparation of probe DNA, which were successfully applied to the detection of host DNA content in recombinant biological products, ensuring the safety of clinical medication sex.
[0044] Condition 1:
[0045] (1) Dilute the genomic DNA standard (136 μg / ml) with purified water to 40 μg / ml, and perform ultrasonic treatment with an ultrasonic instrument. The ultrasonic parameters are shown in Table 4.
[0046] Table 4:
[0047] Genomic DNA concentration
Ultraso...
Embodiment 2
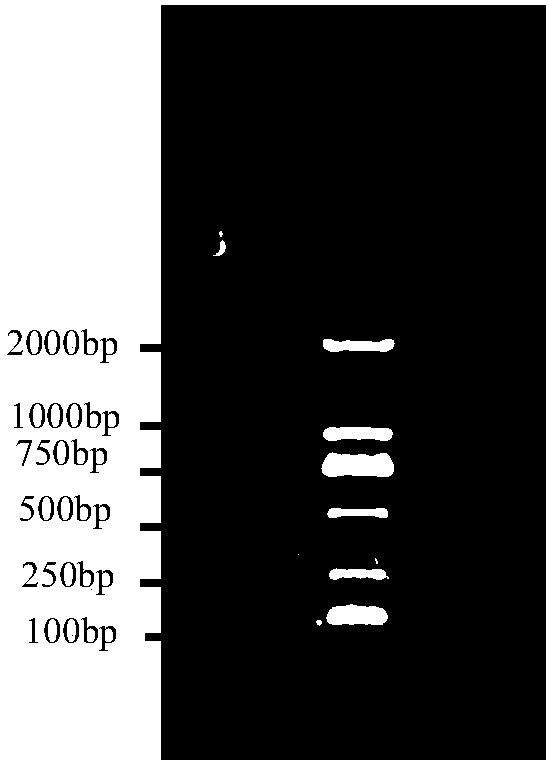
[0092] Example 2 Residual Detection of Recombinant Biological Product Host (Escherichia coli) DNA
[0093] The inventors used the DNA probes prepared under different conditions in Example 1 to detect and compare the amount of host DNA residues in recombinant biological products, in order to study the hybridization efficiency and detection effect of the DNA probes prepared under different conditions, in order to screen out the optimal The method is used for quality control of host DNA residues in recombinant biological products to ensure the safety of clinical medication.
[0094] Condition 1:
[0095] The inventor used the probe DNA prepared in Example 1 condition 1 to detect and analyze the residual amount of recombinant biological product host DNA (derived from the Roche kit), and the detection steps included denaturation and dilution of the test sample and positive control (positive Control gDNA is diluted to 2ug / ml, 0.2ug / ml, 0.02ug / ml with salmon sperm DNA diluent; the t...
Embodiment 3
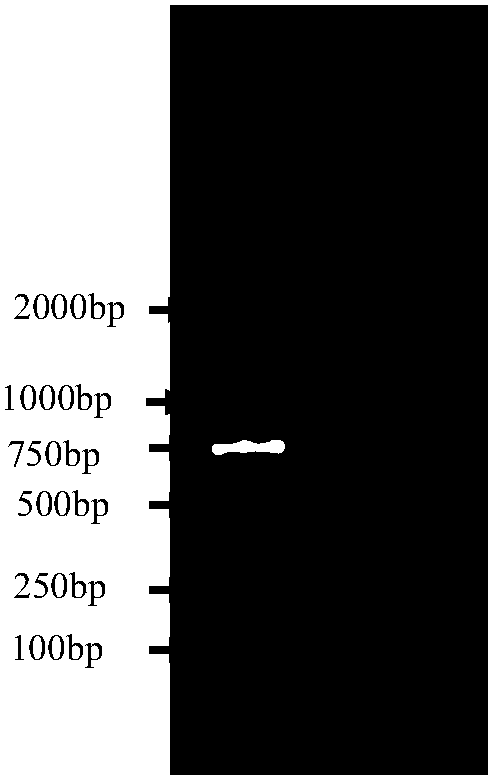
[0110] Example 3 Comparative Study on Sample Diluents in Residual Detection of Recombinant Biological Products Host DNA
[0111] When the inventor was developing the method in the early stage, the positive control gDNA and the test product were diluted with purified water or TE buffer solution, resulting in distortion of the test results, and the hybridization spots were not in the same color system, which could not be judged, so it is not suitable for biological products. Quantitative determination. After screening and optimization in the later stage, the inventor found that the salmon sperm DNA diluent was used for dilution, and the probe DNA prepared under condition 3 or 4 of Example 1 was used for hybridization, and the test results were stable and reliable. The results of using different dilutions in the determination of the residual amount of biological product host DNA are as follows: Figure 9 shown. Figure 9 The results showed that when the positive control gDNA an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com