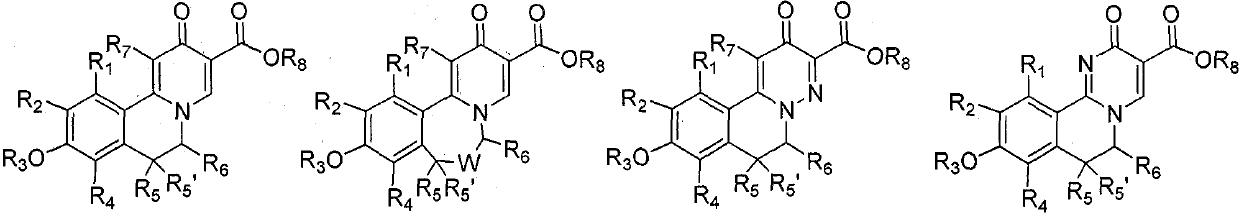
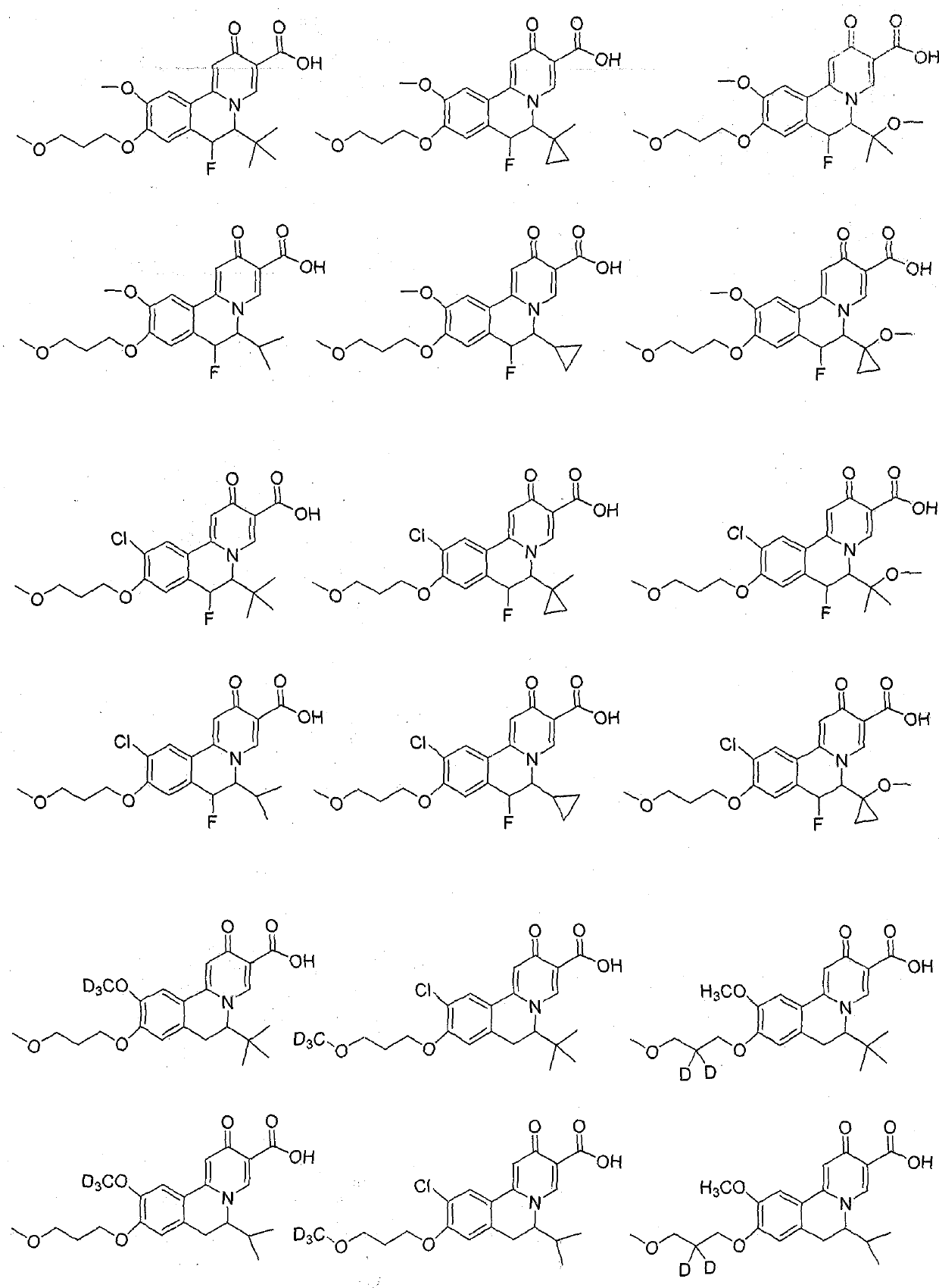
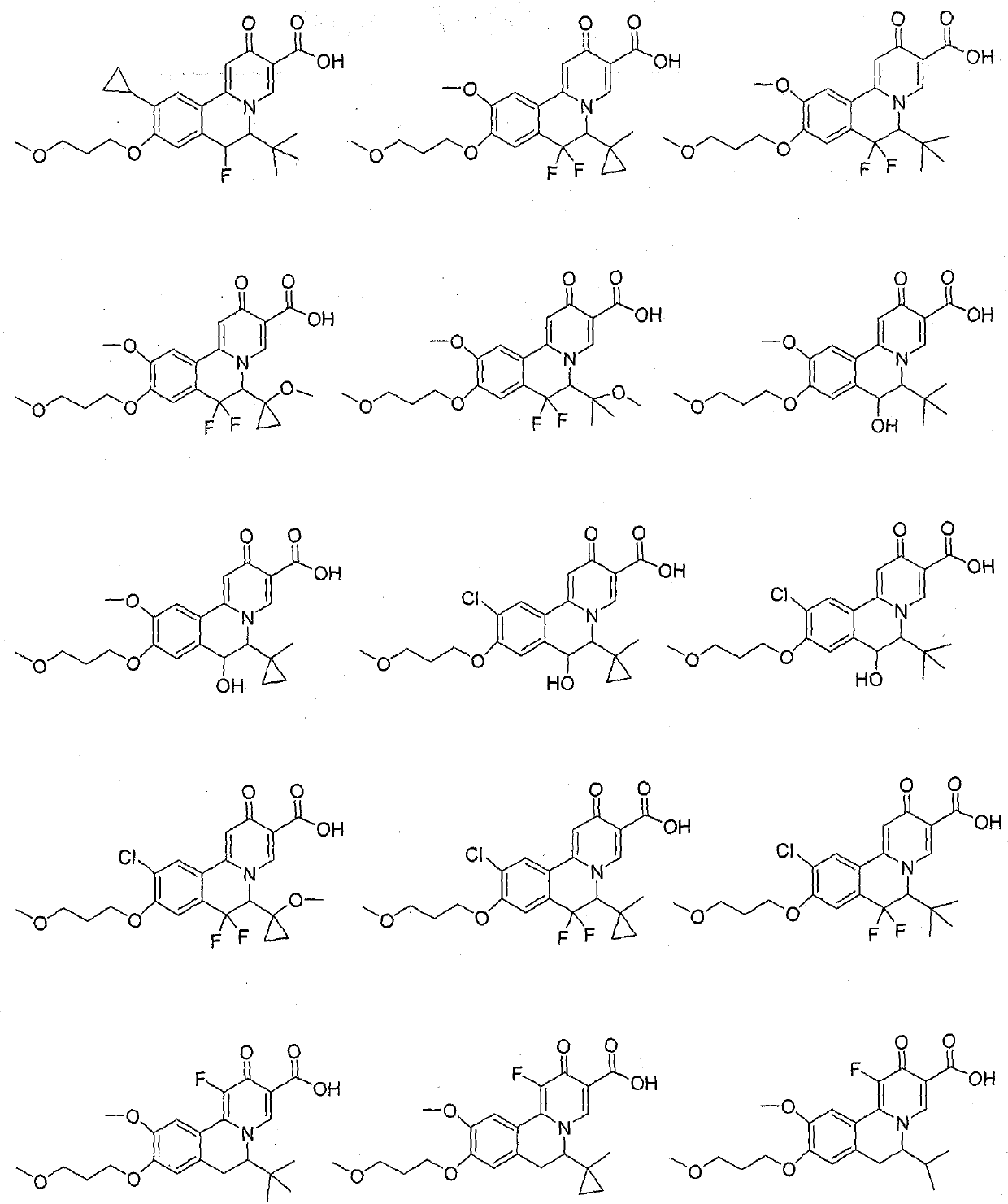
Isoquinoline compound, medicinal composition thereof and application of isoquinoline compound as antiviral drug
A technology of isoquinoline and compound, applied in the field of hepatitis B surface antigen inhibitor and hepatitis B DNA inhibitor, in the field of treatment and/or prevention of infection with hepatitis B virus, and can solve the problem of inability to reduce hepatitis B surface antigen and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: 10 Chloro-1-fluoro-6-isopropyl-9-(3-methoxypropoxy)-2-oxo-6,7-dihydro-2H-pyrido[2,1 -α]isoquinoline-3-carboxylic acid (9)
[0062]
[0063] Modified Step 1: Synthesis of 4-bromo-1-chloro-2-(2-methoxypropoxy)benzene (2)
[0064]
[0065] 1-bromo-2-methoxypropane (49.8g, 325.4mmol) was added dropwise to 5-bromo-2-chlorophenol (22.5g, 108.4mmol) and anhydrous potassium carbonate (45g, 325.6mmol) in DMF (30ml ) suspension, stirred overnight at room temperature, poured into water, extracted three times with ethyl acetate, combined organic phases, washed with water and saturated brine, dried over anhydrous sodium sulfate, concentrated to obtain a yellow oily liquid, 27g, 89%. ESI-MS: [M+H]+: 278.9.
[0066] Modified Step 2: 1-(4-Chloro-3-(2-methoxypropoxy)phenyl)-3-methylbutan-2-one (3)
[0067]
[0068] To THF (180 mL) was added compound 2 (21.0 g, 75.1 mmol), Pd2(dba)3 (1.38 g, 1.5 mmol), xantphos (1.74 g, 3.0 mmol), sodium tert-butoxide (13.0 g, 135....
Embodiment 2
[0089] Example 2: 10-chloro-6-isopropyl-9-(2-methoxypropoxy)-1-methyl-2-oxo-6,7-dihydro-2H-pyrido[2 , 1-α]isoquinoline-3-carboxylic acid (12)
[0090]
[0091] Step 1: 10-Chloro-6-isopropyl-9-(2-methoxypropoxy)-1-methyl-2-oxo-1,6,7,11b tetrahydro-2H-pyrido Synthesis of ethyl [2,1-α]isoquinoline-3-carboxylate (10)
[0092]
[0093] Compound 6 (5g, 16.9mmol), ethyl (E)-2-(ethoxymethylene)-3-oxopentanoate (10.6g, 52.9mmol) in ethanol (50mL) was heated at 100°C, Reacted overnight, concentrated, and simply purified to obtain compound 10, 3.7 g, 48%. Proceed directly to the next reaction. ESI-MS: [M+H]+: 450.2.
[0094] Step 2: 10-Chloro-6-isopropyl-9-(2-methoxypropoxy)-1-methyl-2-oxo-6,7-dihydro-2H-pyrido[2, Synthesis of ethyl 1-α]isoquinoline-3-carboxylate (11)
[0095]
[0096] Compound 10 (3g, 6.6mmol) and p-chlorobenzoquinone (1.63g, 6.6mmol) were dissolved in DME (20mL), heated to 70°C, reacted under nitrogen for 3 hours, poured into water, extracted with dichlo...
Embodiment 3
[0102] Example 3: 10-chloro-1-cyano-6-isopropyl-9-(2-methoxypropoxy)-2-oxo-6,7-dihydro-2H-pyrido[2 , 1-α]isoquinoline-3-carboxylic acid (17)
[0103]
[0104] Step 1: 10-Chloro-6-isopropyl-9-(2-methoxypropoxy)-2-oxo1,6,7,11b tetrahydro-2H-pyrido[2,1-α ] Synthesis of ethyl isoquinoline-3-carboxylate (13)
[0105]
[0106] Compound 6 (3g, 10.1mmol), ethyl (E)-2-(ethoxymethylene)-3-oxobutanoate (5.94g, 31.9mmol) in ethanol (30mL) was heated at 100°C, Reacted overnight, concentrated, and simply purified to obtain compound 13, 2.23g, 50.4%. Proceed directly to the next reaction. ESI-MS: [M+H]+: 436.1.
[0107] Step 2: 10-Chloro-6-isopropyl-9-(2-methoxypropoxy)-2-oxo-6,7-dihydro-2H-pyrido[2,1-α]iso Synthesis of ethyl quinoline-3-carboxylate (14)
[0108]
[0109] Compound 13 (2.0g, 4.5mmol) and chlorbenzoquinone (1.16g, 4.7mmol) were dissolved in DME (15mL), heated to 70°C, reacted under nitrogen for 3 hours, poured into water, extracted with dichloromethane, water an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com