Modified Chinese hamster ovary (CHO) cells and application thereof
A cell and cell line technology, applied in the fields of cell engineering and genetic engineering, can solve problems such as the inability of cells to grow and limited production capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1: Screening and Characterization of CHO-9618s Cell Line
[0118] 1.1. Screening of CHO-K1 cell line (CHO-9618s) capable of high-density growth under serum-free and suspension conditions
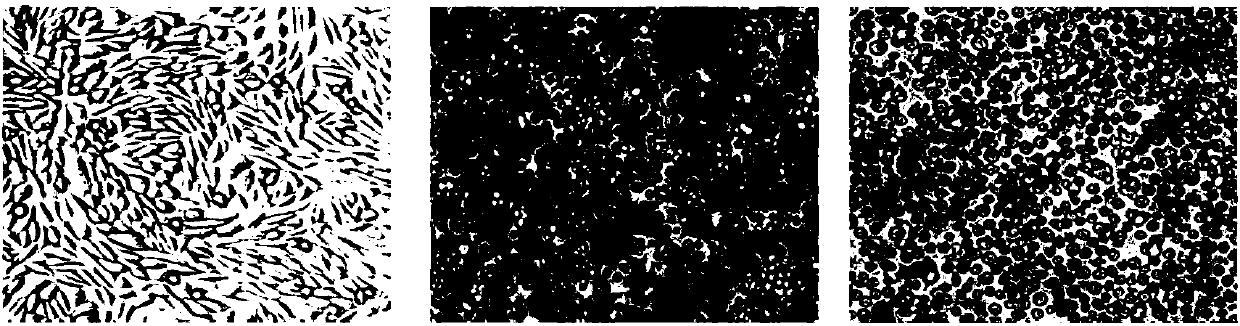
[0119] The CHO-K1 cell line (ATCC CRL-9618) purchased from ATCC was cultured adherently in DMEM / F12 medium (abbreviated as DF12 medium) supplemented with 10% FBS, and the CHO in the logarithmic growth phase was selected. - K1 cells. The cultured cells were observed through a microscope. The results showed that the CHO-K1 cells were in the shape of willow leaves and grew well on the wall ( figure 1 A).
[0120] The medium was replaced with a 1:1 mixture of DF12 medium and SFM IV medium (the final concentration of FBS was 5%), and the cells were continued to be cultured statically for at least 1 week. Then, replace the medium with a 1:4 mixture of DF12 medium and SFM IV medium (the final concentration of FBS is 2%), and continue to culture the cells statically until the cell...
Embodiment 2
[0131] Construction and characterization of embodiment 2.CHO-NIDVD
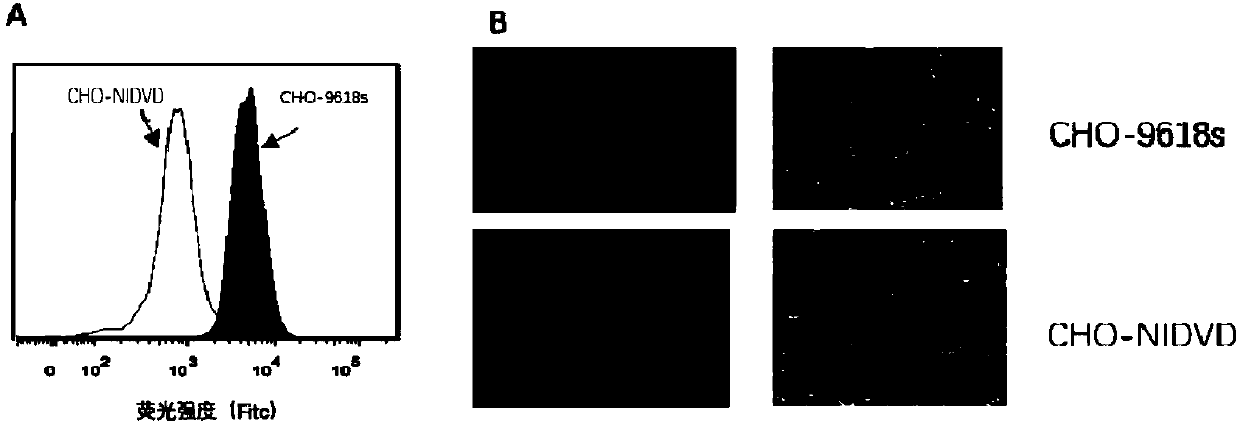
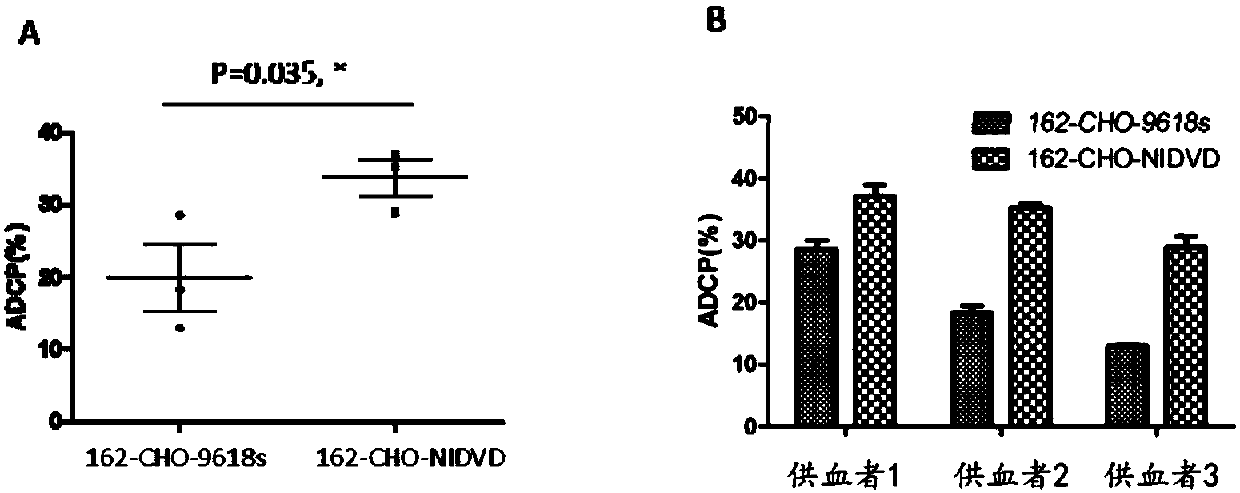
[0132] In this example, the cell line CHO-9618s was modified to construct a new cell line CHO-NIDVD, in which the GS gene and the FUT8 gene had been knocked out.
[0133] 2.1. Construction of CHO-NIDVD cell line
[0134] Briefly, the cell line CHO-9618s was modified according to the gene editing method described in Ran, F.A. et al. Genome engineering using the CRISPR-Cas9system. Nature protocols 8(143):2281-2308 (2013); The gene Zeocin (Zeo) replaces the fifth exon of the GS gene to achieve knockout of the GS gene; and, introduces a frameshift mutation (ie, inserts a base) in the seventh exon of the FUT8 gene , to achieve knockout of the FUT8 gene. The nucleotide sequence of the fifth exon of the GS gene (NCBI accession number: NW_003613921.1; positions 1430036-1435423) is shown in SEQ ID NO:1. The nucleotide sequence of the seventh exon of the FUT8 gene (NCBI accession number: NW_003613860.1; positions 60...
Embodiment 3
[0149] Example 3. Transient expression of foreign proteins in CHO-NIDVD
[0150] The expression plasmid PTT5-GFP encoding green fluorescent protein was transfected into CHO-NIDVD cells in logarithmic growth phase. After 48 hours, observe and take pictures under a fluorescent microscope. Observation results such as Figure 13 shown. The results showed that CHO-NIDVD can be used to express foreign proteins (such as green fluorescent protein).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com