Melitracen hydrochloride crystal form A and preparation method thereof
A technology of melitracen hydrochloride and melitracen hydrochloride, which is applied in the field of medicinal chemistry, can solve the problems affecting the efficacy and safety of drugs, different melting points, solubility and stability, affecting the absorption and release of drugs, etc., and achieves suitable industrial production. , good stability and solubility, good druggability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
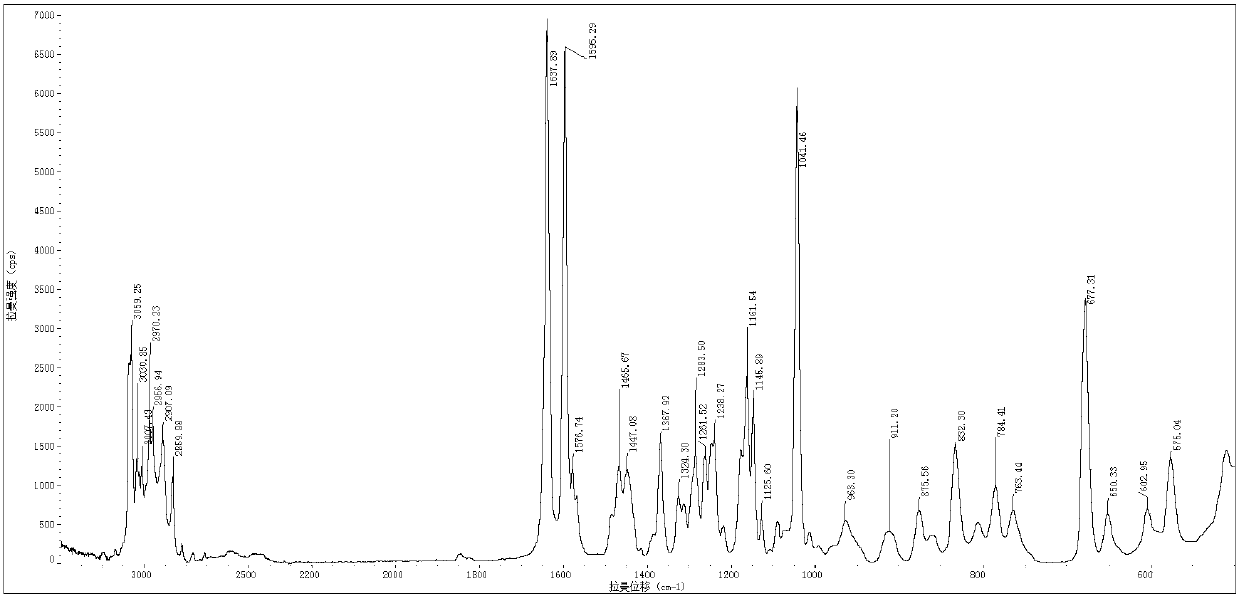
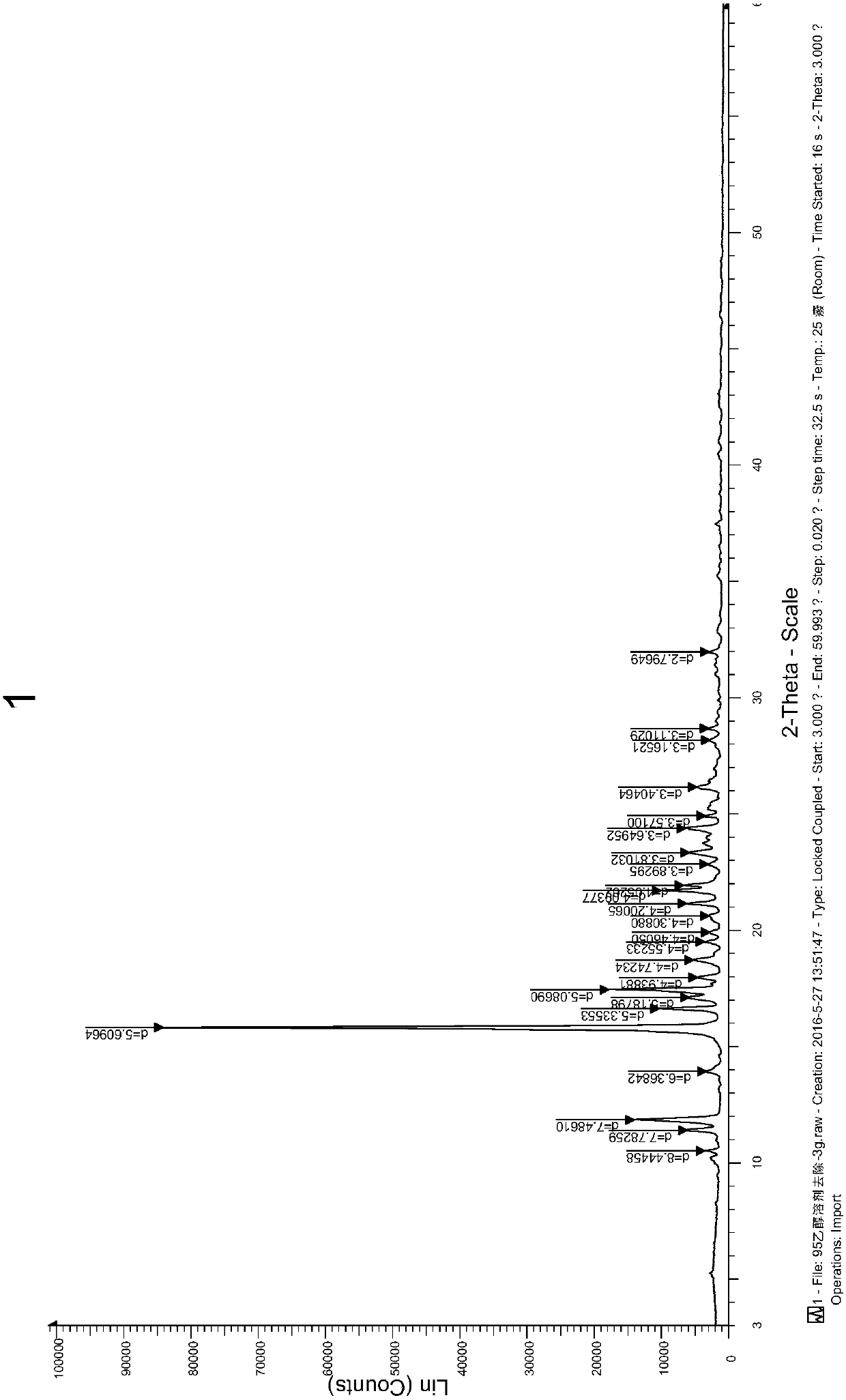
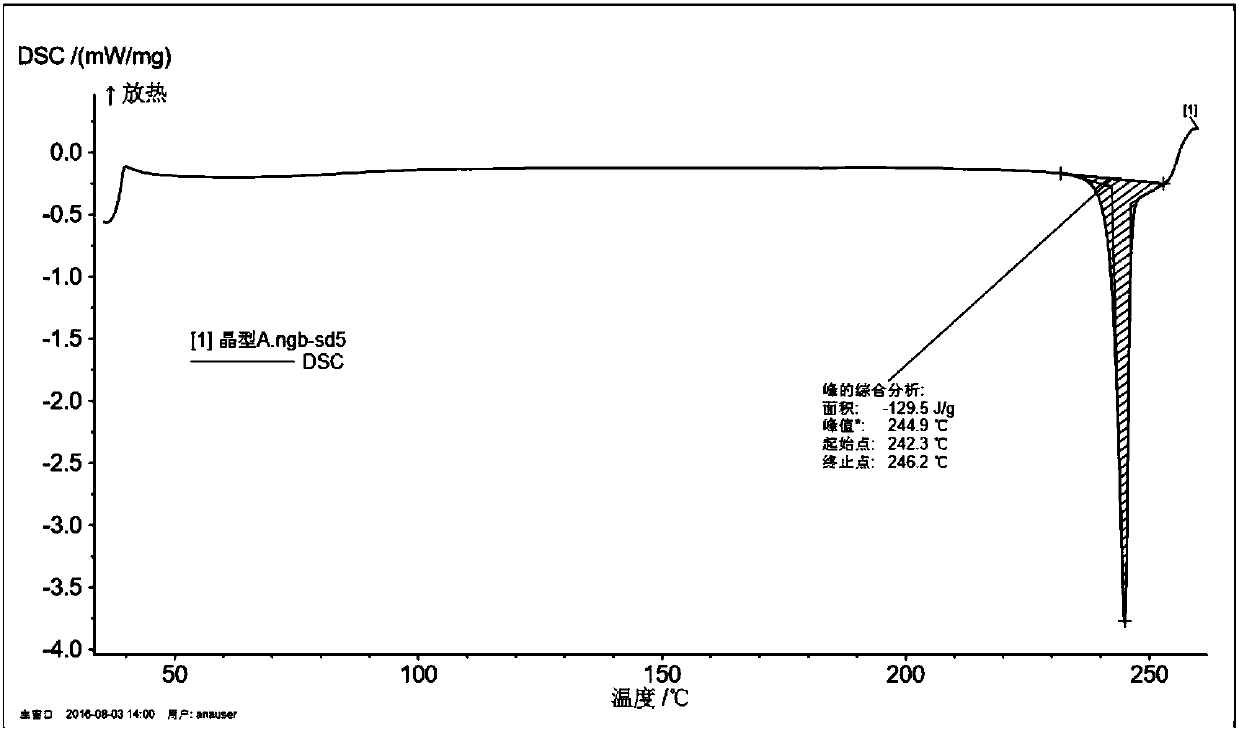
[0050] Weigh 1 g of melitracen hydrochloride sample, put it into an eggplant-shaped bottle, add 10 ml of 95% ethanol, and remove the solvent by rotary evaporation under reduced pressure at a vacuum of 0.09 MPa and 45°C, collect the solid, and dry it overnight at 60°C to obtain melitracen hydrochloride Trisine crystal form A. The purity is 99.8%, X-ray powder diffraction as figure 1 shown.
Embodiment 2
[0052] Weigh 1 g of melitracen hydrochloride sample, put it into an eggplant-shaped bottle, add 10 ml of 90% ethanol, and remove the solvent by rotary evaporation at a vacuum of 0.09 MPa and 60°C, collect the solid, and dry it overnight at 50°C to obtain melitracen hydrochloride. Trisine crystal form A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com