Synthetic method of furan derivatives containing methylthio group
A technology of thiofuran and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of limited application, difficult to obtain 1,4-diketone compounds, etc., and achieves simple steps, is conducive to large-scale production, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~18
[0041] Following examples 1~18 react according to the best reaction condition after above-mentioned optimization:
Embodiment 1
[0043] Raw materials: acetophenone;
[0044] Target product:
[0045] Yield: 83%;
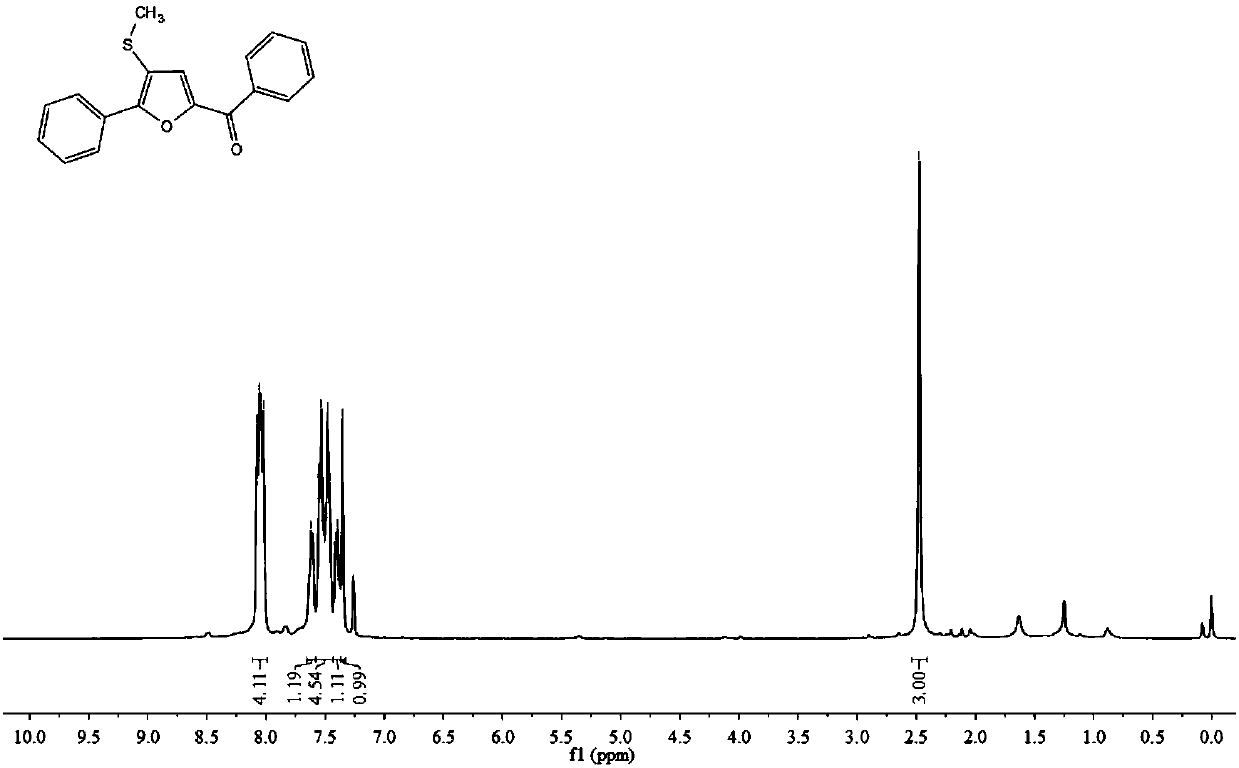
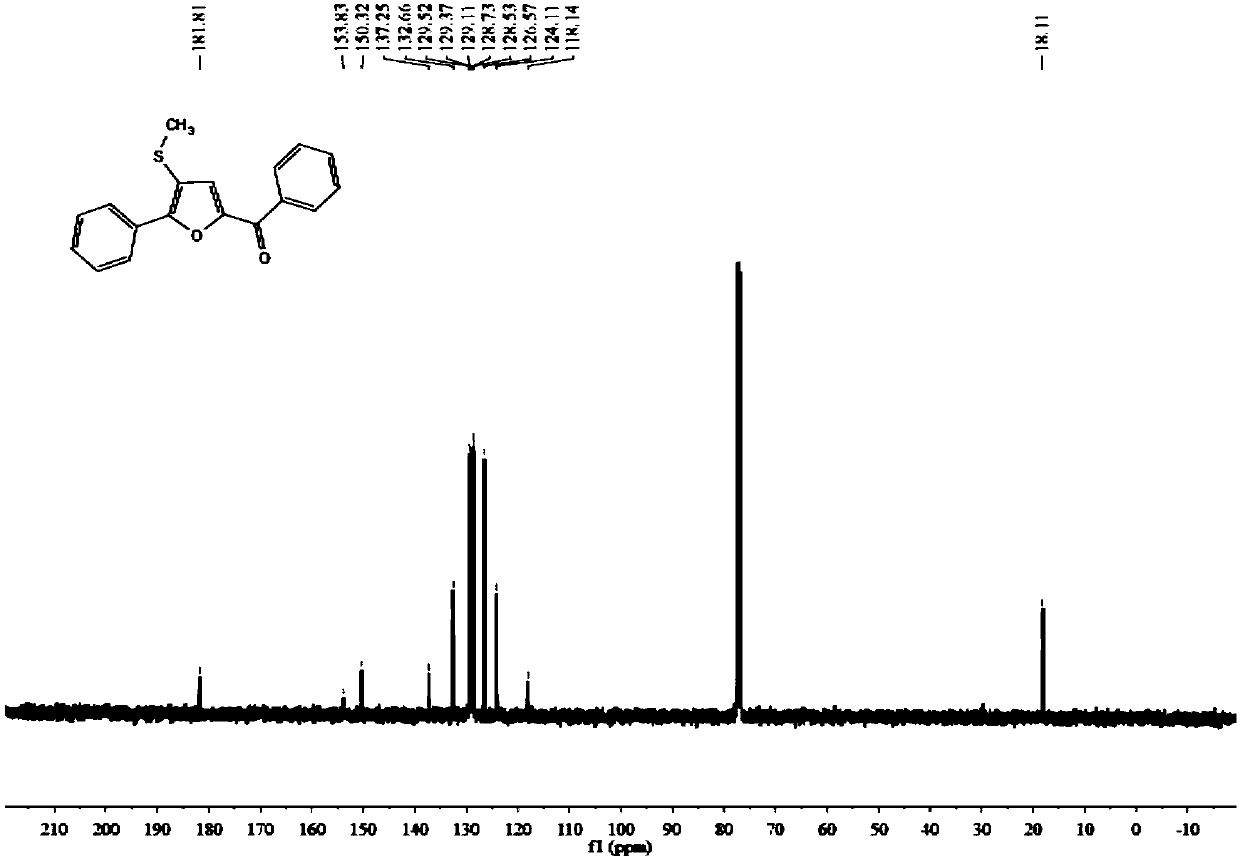
[0046] 1 H NMR (400MHz, CDCl 3 ):δ8.05(dd,J=13.7,7.6Hz,1H),7.65–7.59(m,1H),7.53(t,J=7.1Hz,1H),7.48(t,J=7.1Hz,1H) ,7.43–7.37(m,1H),7.35(s,1H),2.48(s,1H).
[0047] 13 C NMR (101MHz, CDCl 3 ): δ181.8, 153.8, 150.3, 137.2, 132.6, 129.5, 129.3, 129.1, 128.7, 128.5, 126.5, 124.1, 118.1, 18.1.
Embodiment 2
[0049] Raw material: 2-bromoacetophenone;
[0050] Target product:
[0051] Yield: 62%;
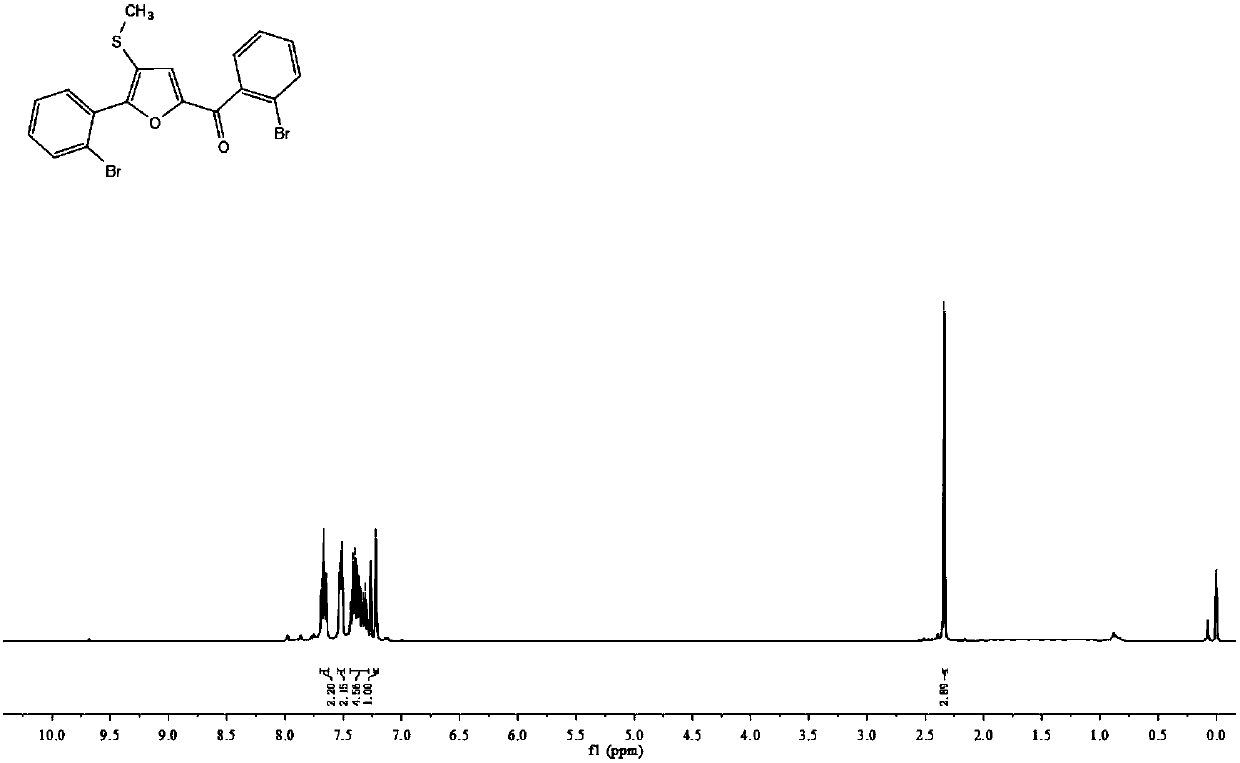
[0052] 1 H NMR (400MHz, CDCl 3 ): δ7.67(t, J=7.7Hz, 1H), 7.52(d, J=7.3Hz, 1H), 7.36(ddd, J=26.3, 15.5, 7.6Hz, 2H), 7.22(s, 1H) ,2.34(s,1H).
[0053] 13 C NMR (100MHz, CDCl 3 ): δ182.4, 155.4, 150.8, 139.1, 133.4, 133.4, 132.3, 131.7, 131.3, 130.1, 129.4, 127.1, 127.1, 123.6, 123.4, 120.9, 120.0, 18.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com