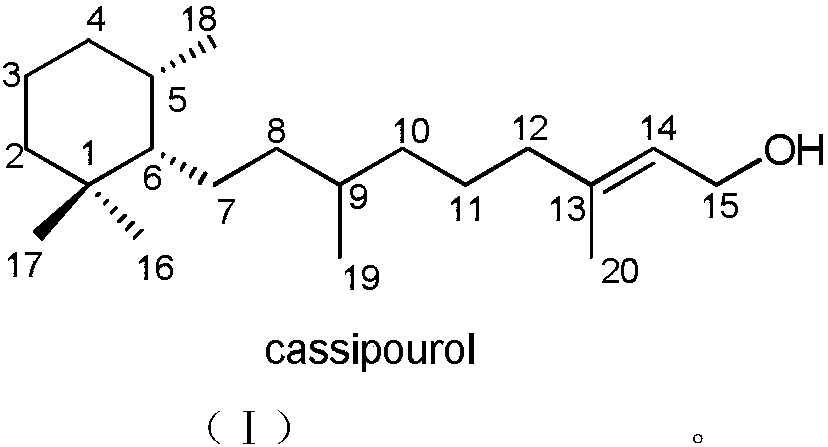
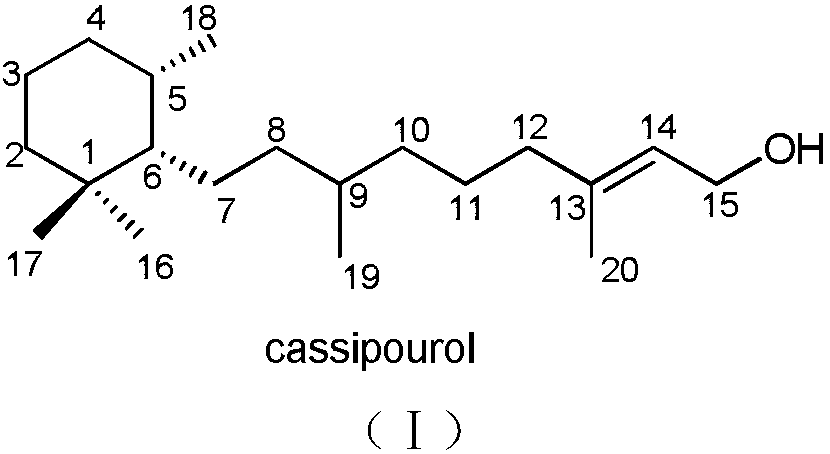
Monocyclic diterpene compound cassipourol, and preparation method and pharmaceutical application thereof
A compound and monocyclic technology, applied in the direction of active ingredients of hydroxyl compounds, organic chemistry, drug combination, etc., can solve the problems that the chemical composition has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Preparation of diterpene compound cassipourol
[0021] Take 300 g of dried branches and leaves of Pinus chinensis, crush them, and extract them by cold soaking with 90% methanol at room temperature for 5 times, combine the extracts, concentrate under reduced pressure, and obtain about 30 g of extract. The extract was dispersed with water and extracted with petroleum ether (PE) and ethyl acetate (EtOAc) successively to obtain 7.0 g of the ethyl acetate part, which was subjected to 100-200 mesh silica gel column chromatography, and the mixture was separated by petroleum ether: ethyl acetate 30:1-0:1 and ethyl acetate:methanol 15:1-0:1 gradient elution to obtain 6 components (Fr.1-Fr.6). Component Fr.1 was further separated by semi-preparative HPLC and eluted isocratically with 90% methanol (flow rate: 3mL / min) to obtain the compound cassipourol (8.0mg, t R = 15.0 min). The NMR and physicochemical data of the compound cassipourol are as follows:
[0022] C...
Embodiment 2
[0023] Example 2: Determination of protein tyrosine phosphatase 1B inhibitory activity
[0024] Experimental method: The genetically recombined hGST-PTP1B-BL21E.coli human PTP1B engineering bacteria was constructed by using the method of molecular biology. The purified hGST-PTP1B recombinant protein can hydrolyze the phospholipid bond of the substrate para-Nitrophenyl Phosphate (pNPP), The obtained dephosphorylated product pNP has a strong light absorption at a wavelength of 405nm, so the change of light absorption at 405nm can be directly detected to observe the change of enzyme activity and the inhibition of the compound to the enzyme activity;
[0025] The percentage inhibition rate of the PTP1B enzyme activity was investigated when the concentration of the compound selected for the primary screening was 20 μg / ml, and the test results showed that the compound cassipourol inhibition rate was higher than 90%;
[0026] Further determination of IC 50 Value: The sample is disso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com