Method for preparing supramolecular platinum-based compounds
A technology of platinum compounds and precipitates, applied in the field of nanotechnology and cancer treatment, can solve the problem of low biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0211] Example 1: Schematic diagram of an exemplary synthetic procedure
[0212]
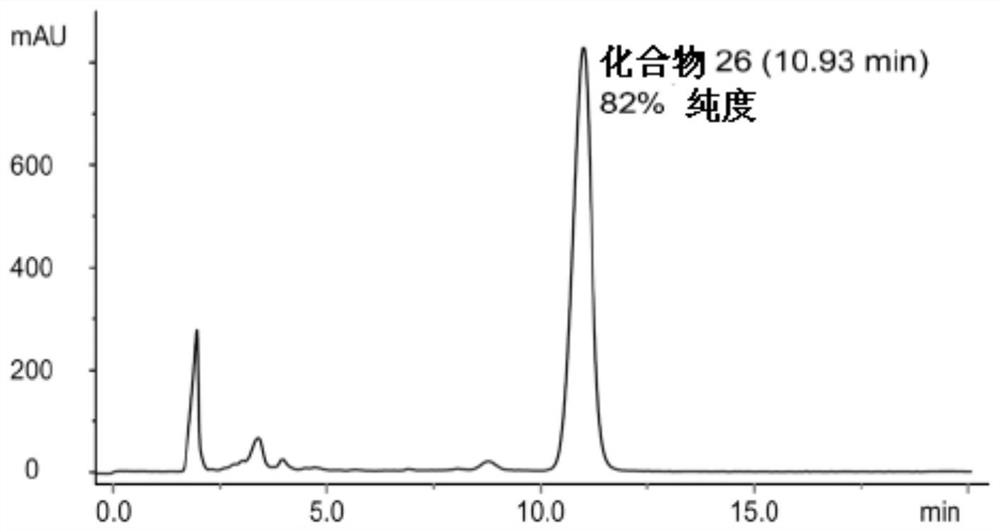
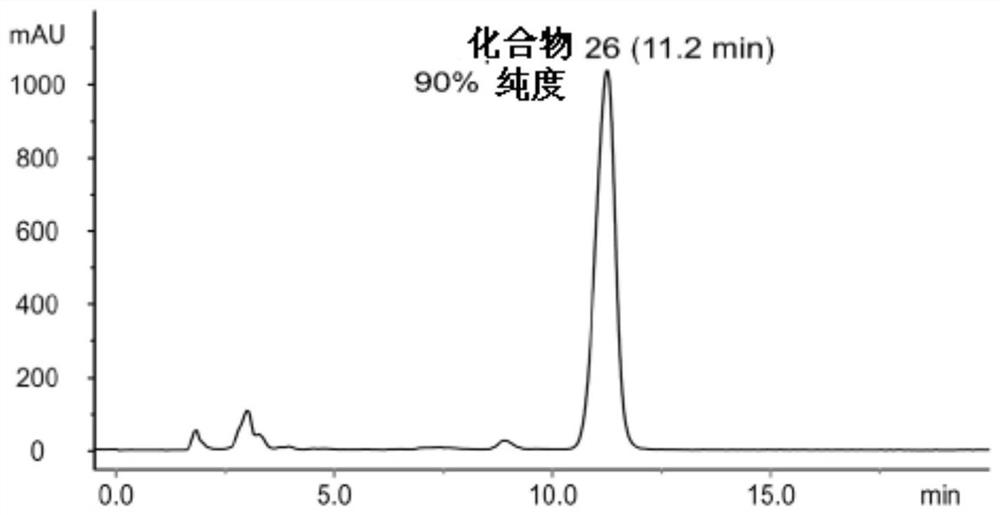
[0213] Similar procedures were used to synthesize compounds 25-28 and other lipid-bound platinum compounds. These exemplary procedures worked well for all of the compounds described. Introducing an additional washing step helped to increase the purity of compound 26 up to 93% (Table 2).
[0214] Table 2: Yield and Purity of Compounds Prepared Using Embodiments of the Invention
[0215]
[0216] figure 1 The HPLC profile of compound 26 before washing with water and acetone is shown, figure 2 The HPLC profile of compound 26 after washing with water and acetone is shown. This is the highest purity achieved by washing.
[0217] Detailed methods for synthesizing the above exemplary compounds are described in International Patent Application PCT / US2014 / 042339 published as WO / 2014 / 201376, which is incorporated herein by reference.
Embodiment 2
[0218] Example 2: Improved synthetic route to intermediate 6 of compound 25
[0219] The multistep synthesis of compound 25 can be found in WO / 2014 / 201376. The reduction in the number of ligand synthesis steps will help improve the yield of compound 25 (API). The procedure referred to involves six steps to reach intermediate 6, whereas the procedure disclosed herein will take two steps to reach the same intermediate. The overall yield of intermediate 6 in the reference procedure (Scheme 2) was 13%. A significant increase (50%) in the yield of intermediate 6 was observed in the new route (Scheme 5).
[0220] Scheme 2: Synthetic steps prior to compound 25
[0221]
[0222] Scheme 3A. Route 1 for the synthesis of intermediate 6 in fewer steps
[0223]
[0224] Scheme 3B. Route 1B for the synthesis of intermediate 6
[0225]
[0226] Experimental details for Scheme 1B (Scheme 3B): 2-Bromoethylamine hydrobromide (5.0 g, 25 mmol) was added portionwise to ethyl bromoace...
Embodiment 3
[0243] Example 3: HPLC purification and analysis
[0244] The lipid-bound platinum compounds of the present disclosure can then be purified by preparative reverse phase HPLC. The compounds can be prepared in C18 stationary phase, NH 2 On-column purification on stationary phase or phenyl stationary phase. Some of the compounds have been found to be unstable on C18, C8, cyano and PFP stationary phases, leading to miscalculations of purity. Therefore, the estimate of purity is based on the NH in which the compound has been found to be stable 2 column or phenyl column.
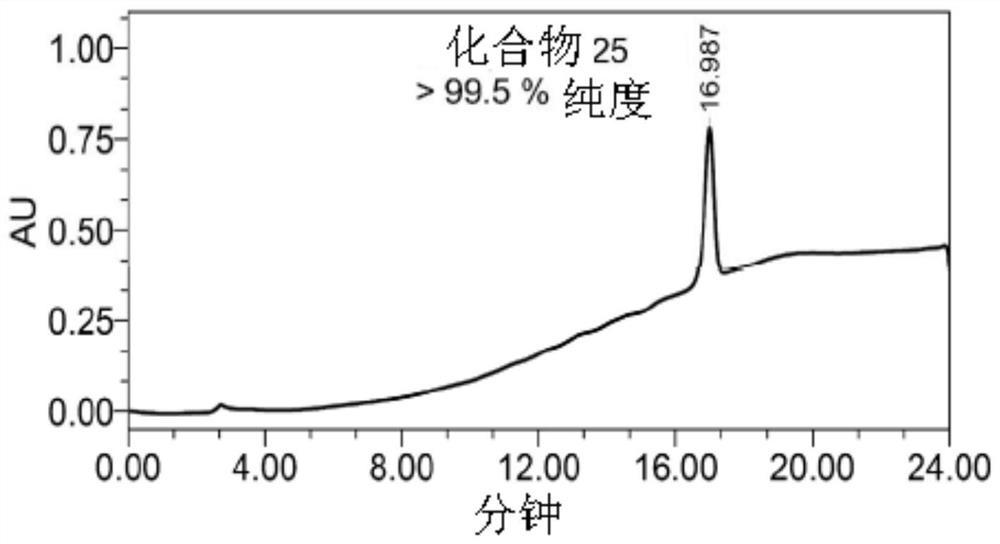
[0245] Purification on a C18 column involved an isocratic method with a mobile phase containing 98% methanol and 2% water. Monitor UV absorbance at 210 nm. Compound peaks were collected, pooled and the solvent removed by evaporation and lyophilization to obtain the compound in powder form with >99.5% purity.
[0246] in NH 2 Or purification on a phenyl column involves a gradient method, starting with a high...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com