A kind of synthetic method and application of helical indole compound based on phenylalanine and polycarbonyl cyclic ketone compound
A technology for indoles and compounds, which is applied in the field of synthesizing spiro indole compounds, can solve the problems of complex synthesis steps, difficulty in operation, high price of reaction raw materials, etc., and achieves cheap and easy-to-obtain raw materials, environment-friendly and pollution-free reaction solvents, and the method. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
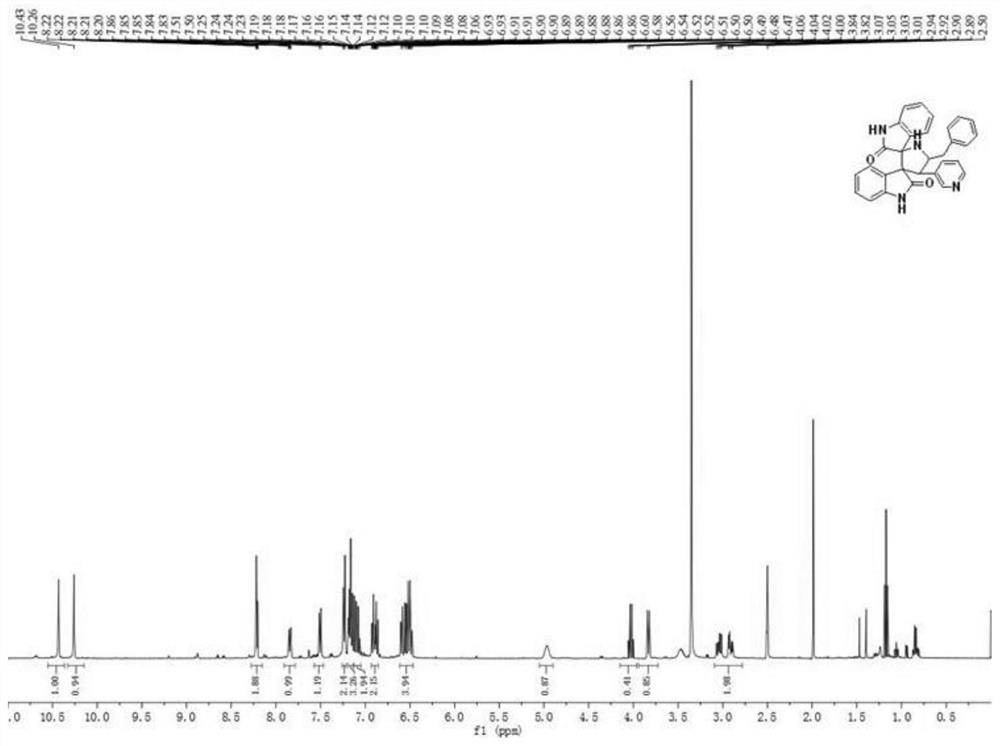
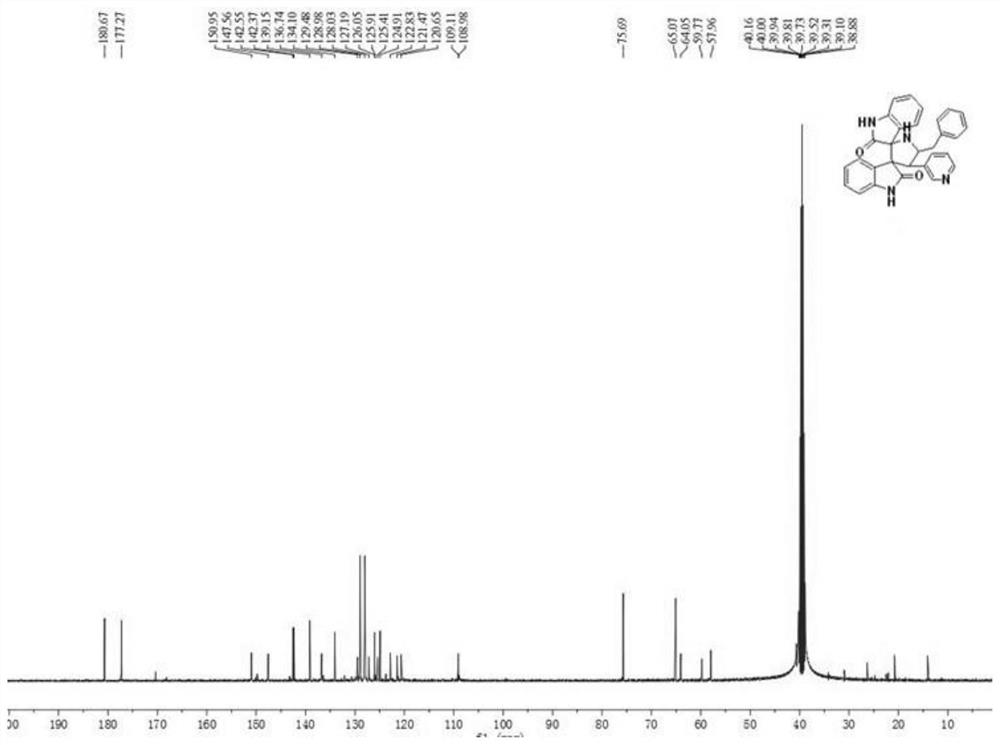
[0037] A kind of synthetic method based on the helical indole compound of phenylalanine, described synthetic method comprises the following steps:
[0038] (1) Weigh 1mmol 2-vinylpyridylindoline, 1mmol phenylalanine and 1mmol isatin respectively, place them in 50mL ethanol aqueous solution reaction solvent, and heat, stir and reflux for 3 hours under air environment;
[0039] (2) After the reaction finishes, wait until the temperature is cooled to room temperature, and the reaction product is suction filtered to obtain a solid crude product, and then the solid crude product is filtered with CH 3 OH / DMF mixed solvent was used for recrystallization, and after recrystallization twice, an orange-yellow solid, that is, pyridyl indoline compound 1 was obtained; the yield was 50%, and the melting point was 120-124°C.
[0040] The product structural formula of gained pyridyl indoline compound 1 is:
[0041]
[0042] H NMR spectrum data of the product:
[0043] 1 H NMR (400MHz, D...
Embodiment 2
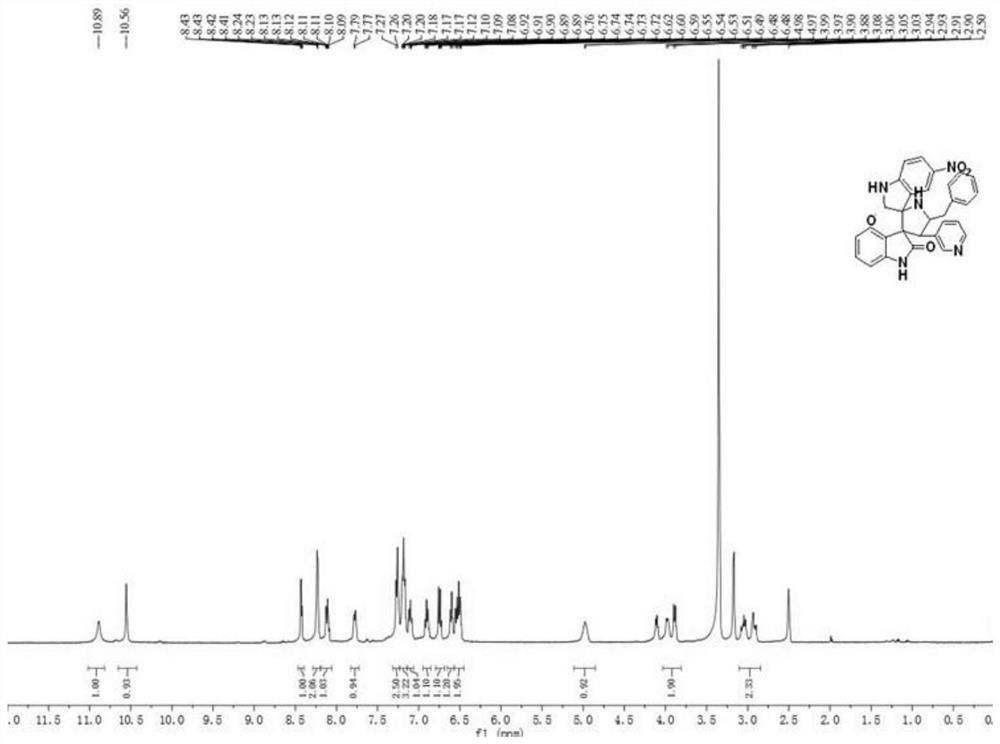
[0047] A kind of synthetic method based on the helical indole compound of phenylalanine, described synthetic method comprises the following steps:
[0048] (1) Weigh 1mmol 2-vinylpyridyl indoline, 1mmol phenylalanine and 1mmol 4-nitro-isatin respectively, place them in 50mL of ethanol aqueous solution reaction solvent, and heat, stir and reflux under air environment for 3 Hour;
[0049] (2) After the reaction finishes, wait until the temperature is cooled to room temperature, and the reaction product is suction filtered to obtain a solid crude product, and then the solid crude product is filtered with CH 3 OH / DMF mixed solvent was used for recrystallization, and after recrystallization twice, a white solid, that is, pyridyl indoline compound 2 was obtained; the yield was 35%, and the melting point was 80-82°C.
[0050] The product structural formula of gained pyridyl indoline compound 2 is:
[0051]
[0052] H NMR spectrum data of the product:
[0053] 1 H NMR (400MHz, ...
Embodiment 3
[0057] A kind of synthetic method based on the helical indole compound of phenylalanine, described synthetic method comprises the following steps:
[0058] (1) Weigh 1mmol 2-vinylpyridylindoline, 1mmol phenylalanine and 1mmol acenaphthenequinone respectively, place them in 50mL ethanol aqueous solution reaction solvent, and heat, stir and reflux for 2 hours under air environment;
[0059] (2) After the reaction finishes, wait until the temperature is cooled to room temperature, and the reaction product is suction filtered to obtain a solid crude product, and then the solid crude product is filtered with CH 3 OH / DMF mixed solvent was used for recrystallization, and after recrystallization twice, an orange-yellow solid, that is, pyridyl indoline compound 3 was obtained; the yield was 63%, and the melting point was 86-87°C.
[0060] The product structural formula of gained pyridyl indoline compound 3 is:
[0061]
[0062] H NMR spectrum data of the product:
[0063] 1 H NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com