Polypeptide inhibitor for REG gamma-20S proteasome and applications thereof
A protein and fusion protein technology, applied in the fields of cell chemistry, molecular biology and biomaterials, can solve the problems of off-target toxicity and poor bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
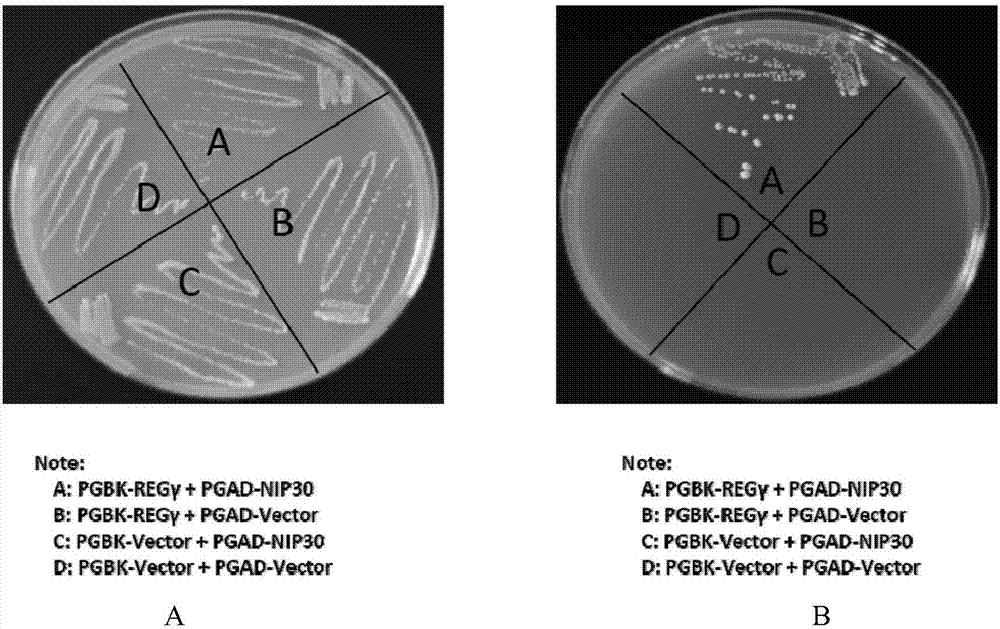
[0081] Example 1 Yeast two-hybrid experiment proves that REGγ is combined with NIP30
[0082] In the present invention, REGγ and NIP30 are respectively constructed on pGBK and pGAD vectors, and used in yeast two-hybrid experiments to prove the interaction between the two.
[0083] The results of yeast two-hybrid figure 1 As shown in A and 1B, yeast colonies grew on the His-Leu-Trp-Ade four-deficient yeast culture plate when pGBK-REGγ was co-transfected with pGAD-NIP30, unlike on the two-deficient plate. This proves that there is an interaction between NIP30 and REGγ.
[0084] The specific experimental method of yeast two-hybrid experiment:
[0085] 1. Burn the inoculation loop on the outer flame of an alcohol lamp to sterilize it. Then dip the yeast strain that has just been taken out from the -80°C refrigerator and inoculate it on the YPDA yeast solid medium by continuous streaking.
[0086] 2. Place the yeast culture dish in a constant temperature incubator at 30°C, and ...
Embodiment 2
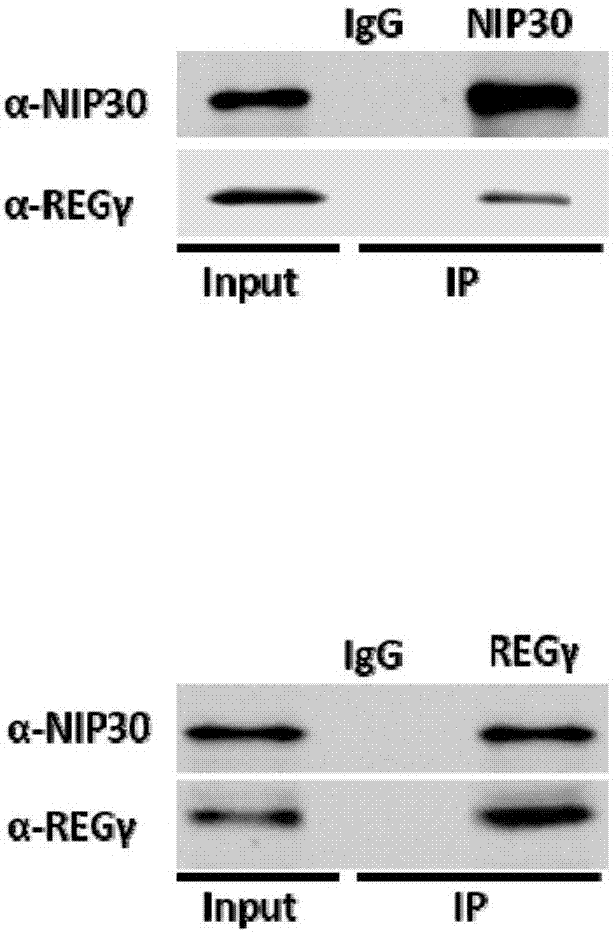
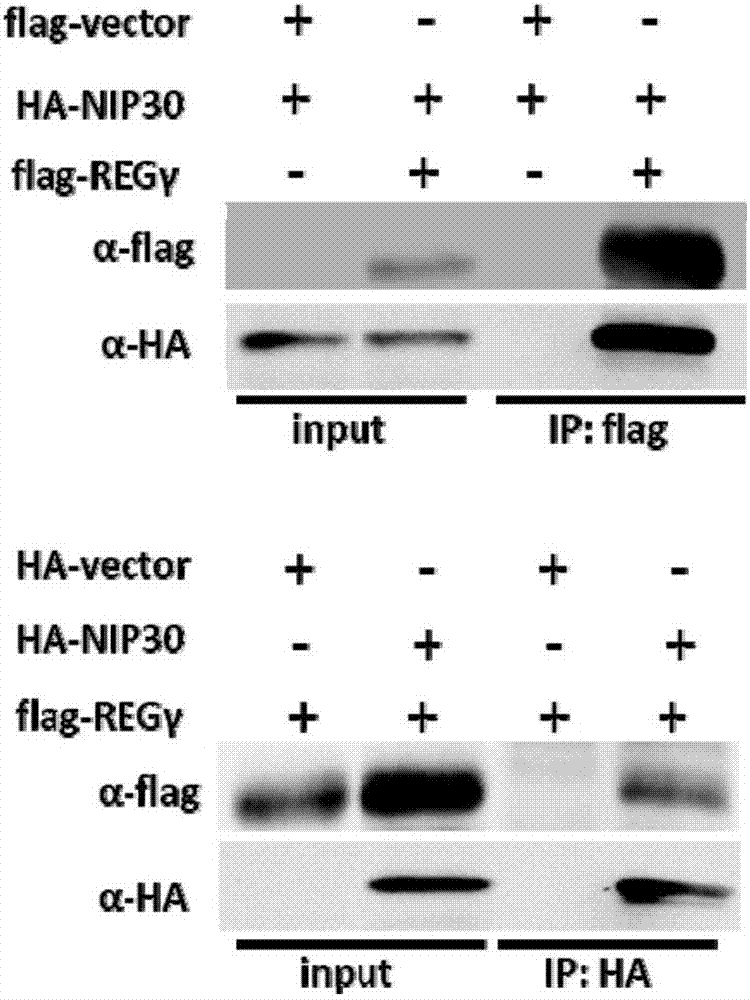
[0098] Example 2 Co-immunoprecipitation experiment proves that REGγ is combined with NIP30
[0099] Example 1 demonstrated the combination of REGγ and NIP30 through yeast two-hybrid experiments. In order to further confirm this combination, the present invention further tested the combination of REGγ and NIP30 by means of co-immunoprecipitation.
[0100] Co-immunoprecipitation specific experimental method:
[0101] (1) Endogenous co-immunoprecipitation
[0102] 1. Collect 293T cells and lyse the cells with 1% NP40 cell lysate for 30 minutes on ice.
[0103] 2. Place the lysed cell lysate in a 4°C centrifuge at 12000 rpm / min, and centrifuge for 15 minutes. Take one-tenth of the cell lysate as Input, then add 20ul of protein loading to lyse, and cook in a water bath for 10 minutes.
[0104] 3. The remaining 9 / 10 cell lysate was divided into two parts, one part was added with 5ul of antibody, the other part was added with 5ul of IgG antibody, and placed in a refrigerator at 4...
Embodiment 3
[0116] Example 3 A variety of cell lines prove that NIP30 has an inhibitory effect on the degradation of P21, a representative substrate of REGγ, and its function depends on the presence of REGγ
[0117] After demonstrating the interaction between REGγ and NIP30 in Example 1 and Example 2, in order to further explore whether NIP30 has a regulatory effect on the function of REGγ, exogenously overexpress NIP30 or use siRNA to knock down NIP30, a representative substrate for REGγ The protein level and RNA level of P21 were detected.
[0118] Specific experimental methods for exogenous transiently overexpressed proteins:
[0119] 1. Transfection was carried out between 8 hours and 24 hours after the cells were fully adherent and stretched after subculture. The cell density to be transfected should preferably be controlled between 50% and 80%, so that the cells are in the logarithmic phase, so as to ensure the transfection efficiency. The main application of the present invention...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com