Dihydroartemisinin tablet and preparation method thereof
A technology for dihydroartemisinin and tablets, which is applied in the field of medicine, can solve the problems of great influence on solubility, difficulty and limitation of dihydroartemisinin, and achieves the guarantee of safety and effectiveness, low impurity content, and dissolution rate. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
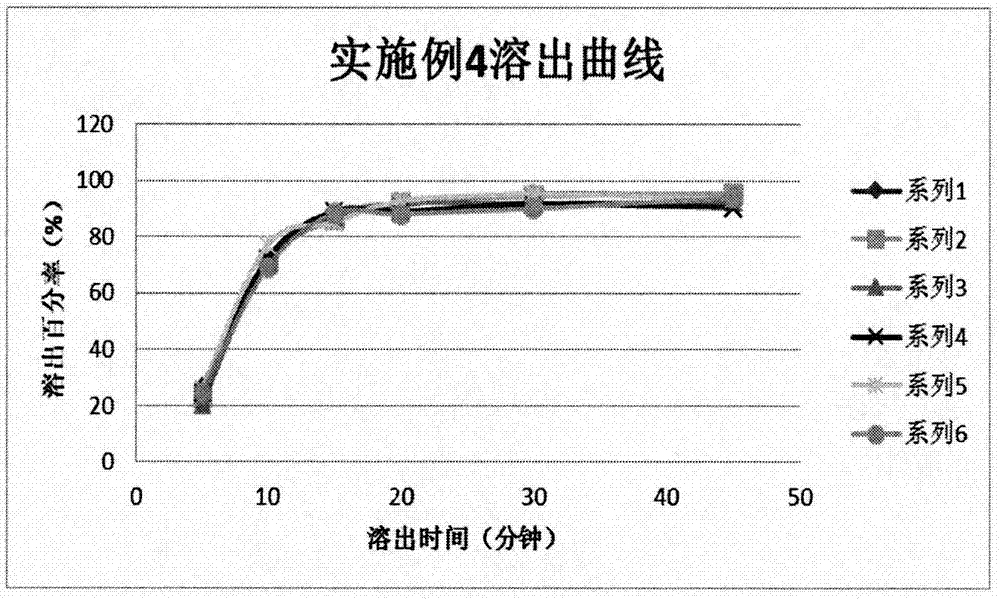
[0039] Example 1: The effect of dry granulation, wet granulation and direct compression of whole powder on the quality of dihydroartemisinin tablets
[0040] 1. Test method
[0041] Weigh the original and auxiliary materials of the prescription to make dihydroartemisinin (D 90 <100μm) contains 10%, lactose (spray dried lactose monohydrate) contains 35%, microcrystalline cellulose (102 or 112) contains 35%, sodium carboxymethyl starch contains 15%, sodium lauryl sulfate contains 1.5% , Polyvinylpyrrolidone contains 3%, magnesium stearate contains 0.5%, and all auxiliary materials pass through an 80-mesh sieve. Weigh 4 parts, and granulate them according to the following methods: the first part is dry-granulated at a pressure of 60kg, and the water outlet on the pressure roller is kept stable under 10℃ during the granulation process; the second part is made with 75% ethanol. The solvent is wet granulated, and the wet granules are dried at 45° C.; the third part is 50% ethanol as the...
Example Embodiment
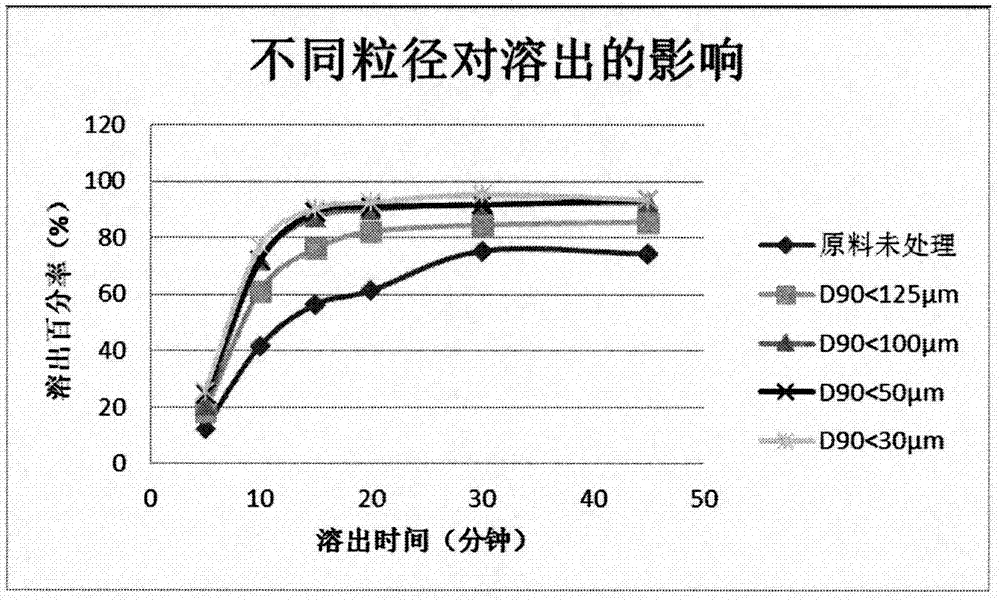
[0056] Example 2: The effect of the particle size of dihydroartemisinin on the dissolution of tablets
[0057] 1. Test method
[0058] Weigh the original and auxiliary materials of the prescription amount respectively, so that dihydroartemisinin contains 10%, lactose (spray dried lactose monohydrate) contains 35%, microcrystalline cellulose (102 or 112) contains 35%, and sodium carboxymethyl starch contains 15%, sodium lauryl sulfate 1.5%, polyvinylpyrrolidone 3%, and magnesium stearate 0.5%. Weigh a total of 5 parts, and process the raw material dihydroartemisinin according to the following methods: the first raw material is not sieved, and the second raw material is passed through a 120-mesh sieve (D 90 90 90 90 <30μm, all auxiliary materials pass through 80 mesh sieve. After mixing, dry granulation was carried out at a pressure of 60 kg, and then tableting was carried out after granulation. The parameters of granulation and tableting were consistent. Take the compressed dihydr...
Example Embodiment
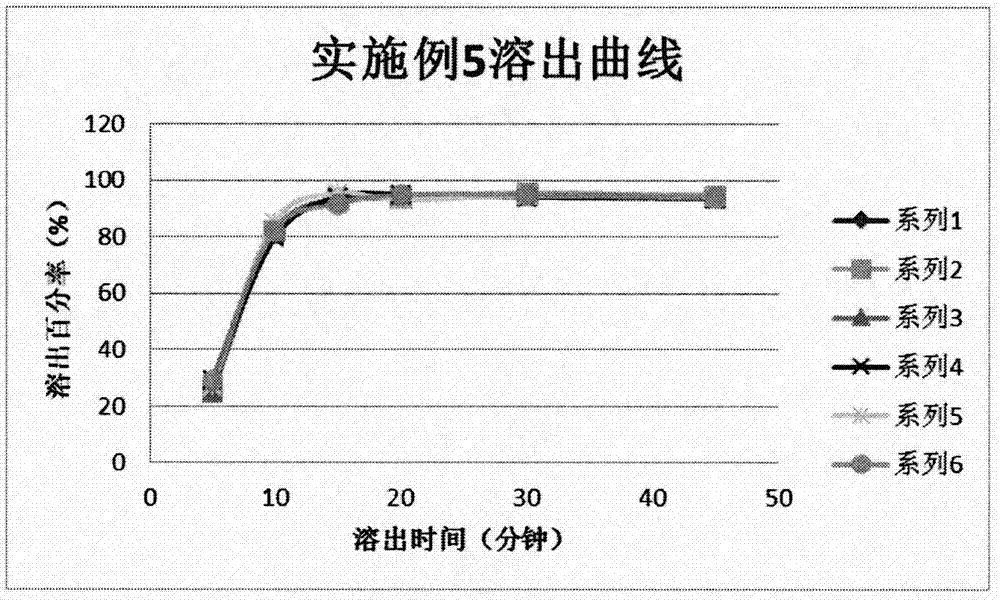
[0066] Example 3: The influence of lactose and microcrystalline cellulose types on the quality of dihydroartemisinin tablets
[0067] 1. Test method
[0068] Weigh the original and auxiliary materials of the prescription to make dihydroartemisinin (D 90 <100μm) contains 10%, lactose (spray-dried lactose monohydrate, Tablettose 80) contains 35%, microcrystalline cellulose (101, 102 or 112) contains 35%, sodium carboxymethyl starch contains 15%, dodecyl Sodium sulfate contains 1.5%, polyvinylpyrrolidone contains 3%, magnesium stearate contains 0.5%, and all auxiliary materials pass through an 80-mesh sieve. Weigh a total of 5 parts, the first part is directly compressed with spray-dried lactose monohydrate, microcrystalline cellulose 101 and other materials in the formulation; the second part is spray-dried lactose monohydrate, microcrystalline cellulose 102 and the formulation The other materials are mixed and directly pressed into tablets; the third part is spray-dried lactose mon...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap