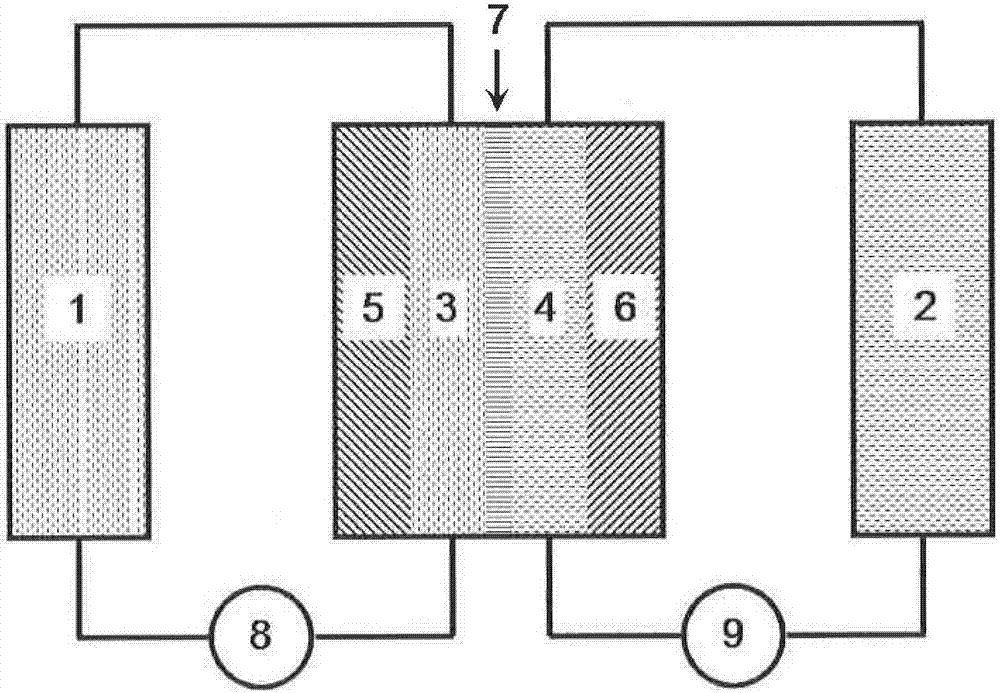
High-power redox flow battery based on the criii/crvi redox couple and its mediated regeneration
A flow battery and battery technology, applied in the field of electrochemical batteries, can solve problems such as electrode scaling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
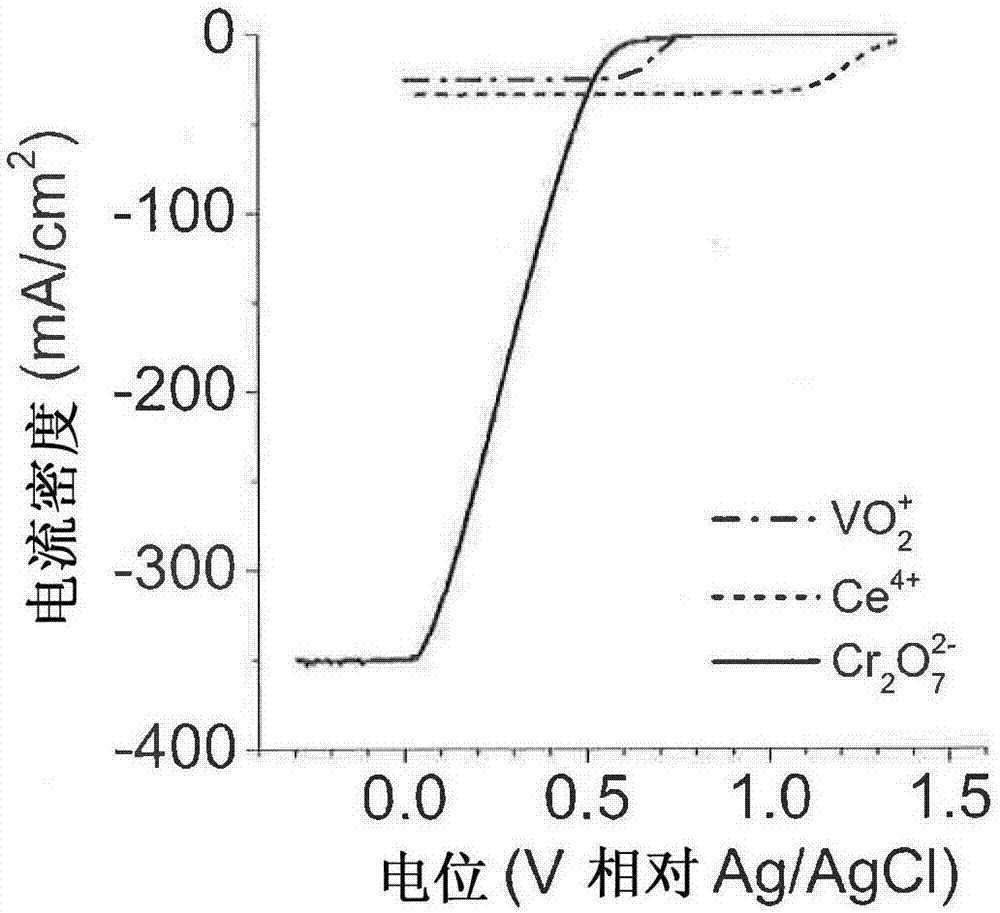
[0093] The involvement of Cr was verified via cyclic voltammetry (CV) in the potential window of +0.2 to +1.5 V vs. Ag / AgCl 3+ with Ce 4+ The reaction produces Ce 3+ EC 催化 mechanism.
[0094] Figure 5 Two cases are shown: (1)5mM Ce on a flat / smooth Au electrode 4+ and 5mM Cr 2 o 7 2- and (2) 5mM Ce 4+ in aqueous 0.5M H 2 SO 4 solution in. exist Figure 5 , solutions (1) and (2) show Ce in the anodic (forward) scan at +1.44 V 3+ The peak of oxidation, where the oxidation current starts above +1.35 V and shows Ce in the cathodic (negative going) scan at +1.35 V 4+ The peak of the reduction, where the net reduction current starts from +1.40V. In CV, there is no Ce 3+ present in bulk solution, while all detected Ce 3+ Both derived from Ce 4+ reduction. In general, the generated Ce 3+ will diffuse into the bulk solution, but the process is slower and there is some Ce in the depletion region near the electrode surface detected in the anodic scan 3+ . Ce 3+ Gen...
Embodiment 2
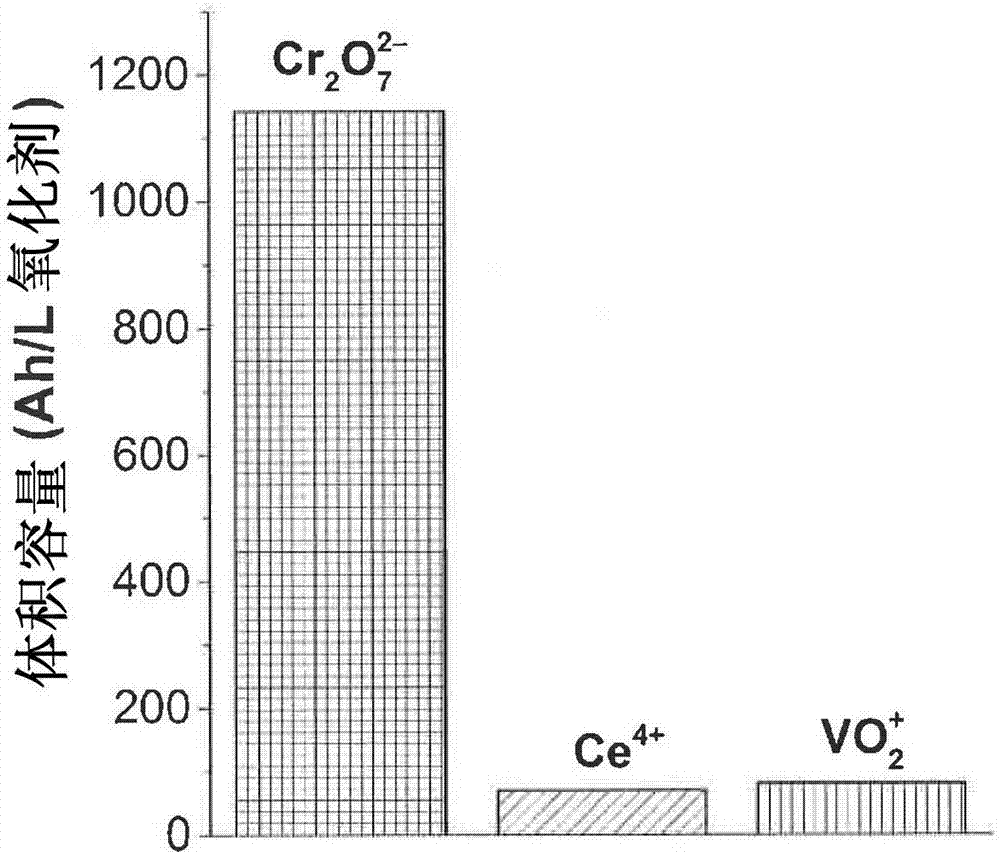
[0098] Cr 2 o 7 2- and Ce 4+ Discharge / charge cycles of the solution confirmed the complete recovery of pristine Cr 2 o 7 2- reduction current. Figure 7 shown at 0.5M H 2 SO 4 2.5mM Cr at 250rpm 2 o 7 2- and 2.5mM Ce 4+ RDE voltammetry, where Cr 2 o 7 2- Reduction occurs at potentials 2 o 7 2- After a (not all) 2.5C discharge at 4000rpm; and (3) Cr 3+ After charging at 2.5C at 4000rpm. The mass-transport-limited currents in (1) and (3) agree, suggesting that pristine Cr 2 o 7 2- complete recovery of concentration.
Embodiment 3
[0100] It was observed that Cr in Demo 3 3+ charging via the expected asymmetric EC 催化 process happens. Figure 8A shows that 2.5C discharge (negative current and charge) occurs rapidly, taking only 11 minutes, while Figure 8B The charging takes 12 hours and 30 minutes. as for EC 催化 The reaction is expected via Ce 4+ Cr 3+ EC 催化 Charging is still faster than only by Ce 3+ Charging Ce 4+ Go faster. This passes at 0.5MH 2 SO 4 Discharge 2.5mM Ce at 4000rpm 4+ solution for 11 minutes ( Figure 8A ) and Ce using the same potential and rpm in Demo 3 (Fig. 8B, inset) 3+ Charge to Ce 4+ And prove. Ce 3+ Oxidation current is the presence of Cr in the solution 3+ 2.5 times of time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com