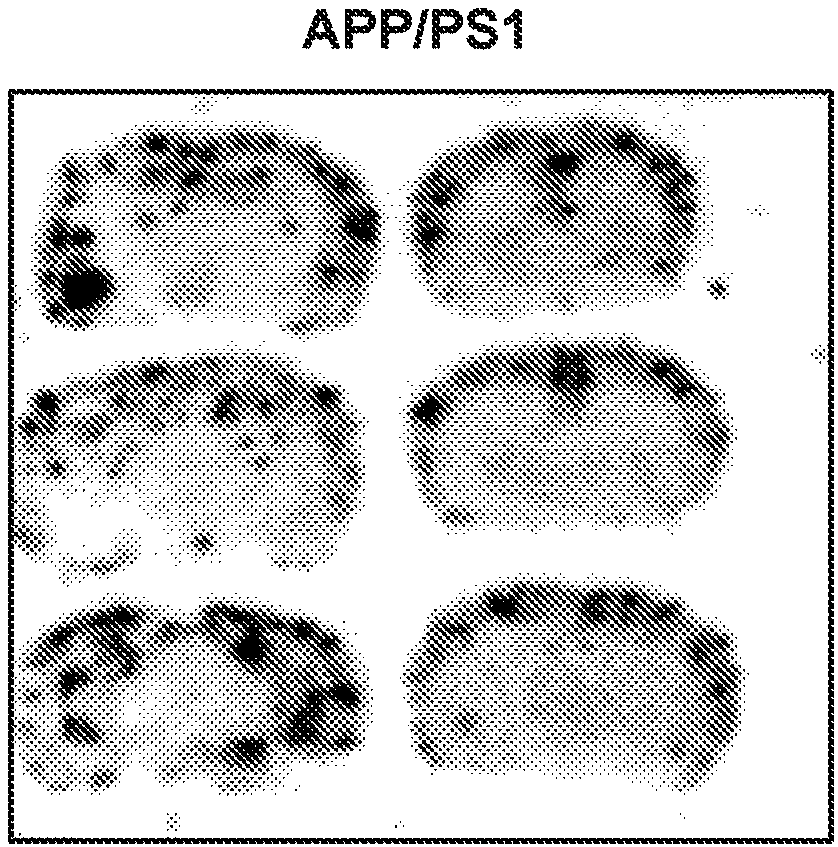
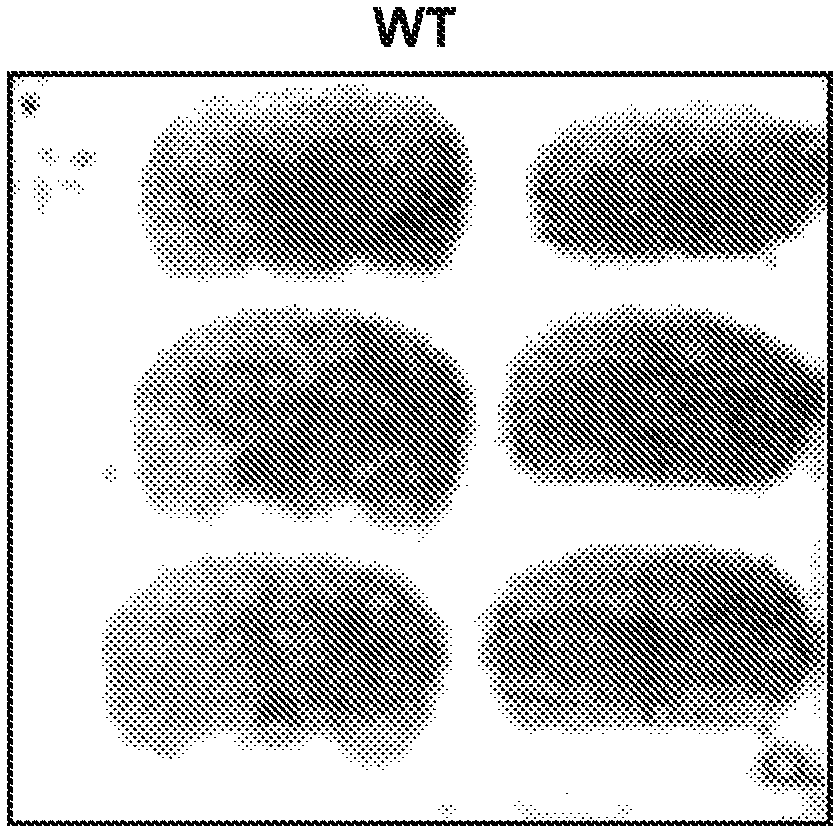
Probes for imaging huntingtin protein
An imaging agent, pharmaceutical salt technology, used in the field of probes for imaging huntingtin protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0476] Example 1, Method 1: 10-Methoxy-N-(pyridin-3-yl)-7-thia-2,5-diazatricyclo[6.4.0.0 2 ,6 ]Dodeca-1(12),3,5,8,10-pentaene-4-carboxamide
[0477] 1 H NMR (500MHz, DMSO) 10.47(s, 1H), 9.04(d, J=2.3Hz, 1H), 8.98(s, 1H), 8.34-8.23(m, 2H), 8.11(d, J=8.9Hz , 1H), 7.73 (d, J=2.5Hz, 1H), 7.47-7.30 (m, 1H), 7.19 (dd, J=8.9, 2.5Hz, 1H), 3.84 (s, 3H). Tr(MET-uHPLC-AB-101)=1.92min, m / z(ES + )(M+H) + 325.
[0478] The following examples were prepared using Method 1 as described above or its analogues.
[0479] Table 1
[0480]
[0481]
[0482]
[0483]
[0484]
[0485] Method 2
[0486] Solution for Method 2
[0487]
[0488] Step 1, Method 2: 10-Hydroxy-7-thia-2,5-diazatricyclo[6.4.0.0 2,6 ]Dodeca-1(8),3,9,11-tetraene-4-carboxylic acid
[0489] 10-methoxy-7-thia-2,5-diazatricyclo[6.4.0.0 2,6 ] Dodeca-1(8),3,9,11-tetraene-4-carboxylic acid (638 mg, 2.57 mmol, prepared by Method 1) was suspended in dichloromethane (50 mL) and stirred for five minutes. 1 ...
Embodiment 1
[0492] Example 1, Method 2: 10-Hydroxy-N-(6-methoxypyridin-3-yl)-7-thia-2,5-diazatricyclo[6.4.0.0 2,6 ]Dodeca-1(8),3,9,11-tetraene-4-carboxamide
[0493] 1 H NMR (500MHz, DMSO) 10.22(s, 1H), 10.08(s, 1H), 8.85(s, 1H), 8.61(d, J=2.6Hz, 1H), 8.15(dd, J=8.9, 2.7Hz ,1H),7.97(d,J=8.8Hz,1H),7.41(d,J=2.3Hz,1H),6.97(dd,J=8.7,2.4Hz,1H),6.82(d,J=8.9Hz ,1H), 3.83(s,3H). Tr(MET-uHPLC-AB-101)=2.48min, m / z(ES + )(M+H) + 341,94%.
[0494] The following examples were prepared using Method 2 as described above:
[0495] Table 2
[0496]
[0497]
[0498] Method 3
[0499] Solution for method 3
[0500]
[0501] Step 1, Method 3: 1,8,10-Triazatricyclo[7.4.0.0 2,7 ]Trideca-2(7),3,5,8,10,12-hexaene-11-carboxylic acid ethyl ester
[0502] 1H-benzimidazol-2-amine (2g, 15.02mmol) and (3E)-4-(dimethylamino)-2-oxobut-3-enoic acid ethyl ester (70%, 4g, 16.36mmol , described in US2011001121) was suspended in acetic acid (50 mL) and the reaction mixture was heated at 120 °C for 16 h....
Embodiment 1
[0507] Example 1, Method 3: N-(6-methoxypyridin-3-yl)-1,8,10-triazatricyclo[7.4.0.0 2,7 ]Trideca-2(7),3,5,8,10,12-hexaene-11-carboxamide
[0508] 1 H NMR (500MHz, DMSO) 11.06(s, 1H), 9.77(d, J=7.0Hz, 1H), 8.71(d, J=2.6Hz, 1H), 8.43(d, J=8.2Hz, 1H), 8.22(dd, J=8.9,2.7Hz,1H),7.96(d,J=8.2Hz,1H),7.77(d,J=7.0Hz,1H),7.64(appt t,J=7.7Hz,1H) , 7.53 (appt t, J = 7.7Hz, 1H), 6.89 (d, J = 8.9Hz, 1H), 3.87 (s, 3H). Tr(MET-uHPLC-AB-101)=2.19min, m / z(ES + )(M+H) + 320.
[0509] The following examples were prepared using method 3 as described above:
[0510] table 3
[0511]
[0512]
[0513]
[0514]
[0515] Method 4
[0516] Solution for method 4
[0517]
[0518] Step 1, Method 4: 10-Methoxy-N-[6-(methylcarbamoyl)pyridin-3-yl]-7-thia-2,5-diazatricyclo[6.4.0.0 2,6 ]Dodeca-1(12),3,5,8,10-pentaene-4-carboxamide
[0519] 10-Methoxy-7-thia-2,5-diazatricyclo[6.4.0.0 in pyridine (2 mL) 2,6 ] dodeca-1(12), 3,5,8,10-pentaene-4-carboxylic acid (95%, 100 mg, 0.38 mmol, de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com