Synthesis of levomethadone hydrochloride or dextromethadone hydrochloride and methods for use thereof
A technology for methadone and levothadone hydrochloride is applied in the synthesis of levomethadone hydrochloride or dexmethadone hydrochloride and its application field, and can solve the problems of reducing the total yield of levomethadone and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
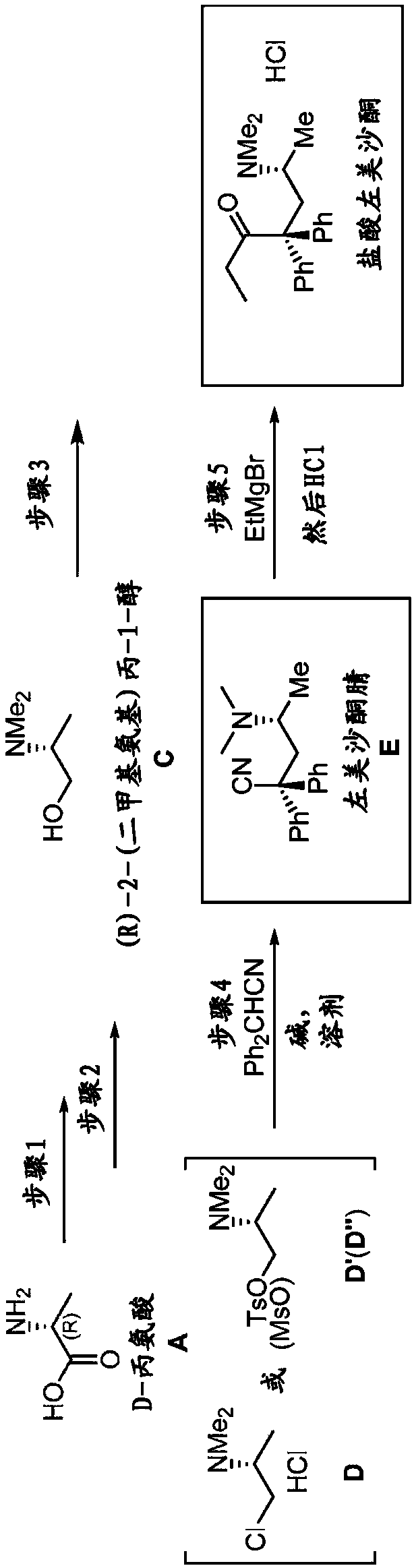
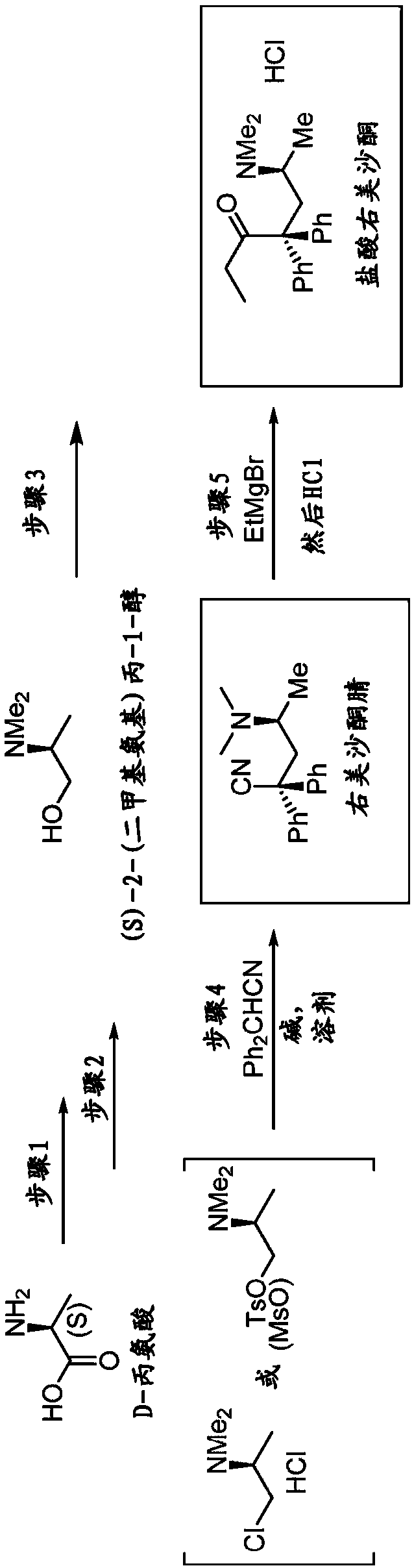
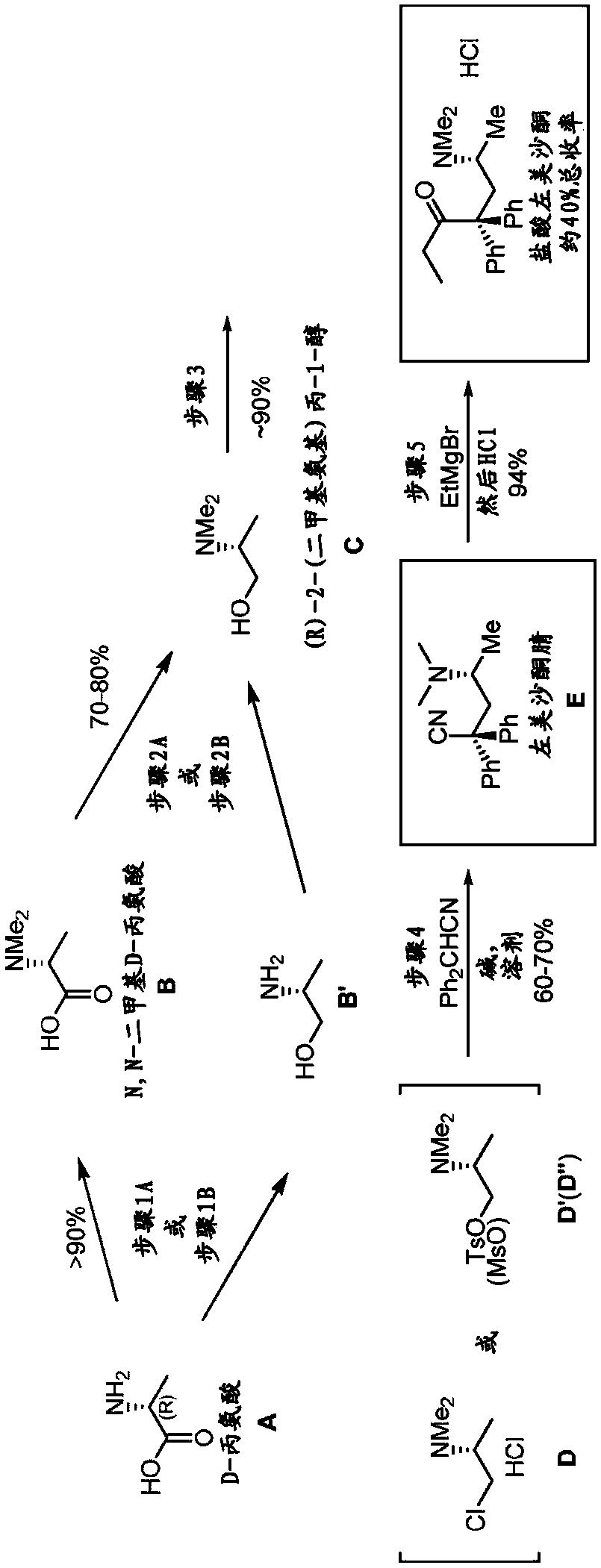
[0310] Example 1.A.N,N-dimethyl D-alanine (B)
[0311] Step 1: Dimethylation of D-alanine (A) by hydrogenation with formaldehyde in the presence of Pd / C to form N,N-dimethyl D-alanine (B)
[0312] This step was performed by a modified literature procedure. See WO 2012 / 162635 and Org Lett 2013, 15, 3118-3121 by Paterson et al., each incorporated by reference.
[0313]Formaldehyde (37%, 125 mL, 1,680 mmol) and Pd / C were added to a solution of D-alanine (A) (50.0 g, 561 mmol) in water (800 mL). The flask was purged with Ar for 10 min, followed by H 2 Purge three times at 50 °C in H 2 The reaction mixture was stirred at (35 psi) for 20 hours. The reaction mixture was then heated to reflux for 1 hour and filtered through a short pad of celite while hot. The filtrate was concentrated under reduced pressure. More water was added (3 times 200 mL each) and the reaction was concentrated to remove unreacted formaldehyde. Residual water was azeotroped with toluene (3 x 100 mL). 1 ...
Embodiment 2
[0316] Example 2. (R)-2-(dimethylamino)propan-1-ol (C)
[0317] Step 2: Reduction of N,N-dimethyl D-alanine (B) with lithium aluminum hydride to form (R)-2-(dimethylamino)propan-1-ol (C)
[0318] At 0 °C, the LiAlH 4 (1.14 g, 30 mmol) was added to a stirred solution of N,N-dimethyl D-alanine (B) (2.34 g, 20 mmol) in THF (30 mL) prepared in step 1 and the resulting reaction mixture was heated to reflux for 16 Hour. After cooling to room temperature, dissolve with 10 mL of saturated NaHCO 3 The reaction was carefully quenched and stirred for 10 minutes to form a thick suspension. Then DCM (50 mL) was added and the organic layer was separated by decantation. DCM (50mL) and anhydrous Na 2 SO 4 (ca. 10 g) was added to the remaining pasty residue and the mixture was stirred vigorously. The DCM layer is then separated. The residue was stirred with two further portions of DCM (50 mL each). use Na 2 SO 4 The combined DCM layers were dried and concentrated under reduced press...
Embodiment 3
[0319] Example 3. (R)-4-(Dimethylamino)-2,2-diphenylvaleronitrile. Step 3: Synthesis of levmethadonenitrile (E) from (R)-2-(dimethylamino)propan-1-ol (C) and diphenylacetonitrile
[0320] At 0°C, 1.0 mL SOCl 2 CHCl 3 (5 mL) solution was carefully added to a solution of crude aminoalcohol C (550 mg, 5.33 mmol) prepared in step 2 in chloroform (5 mL). The reaction mixture was stirred at room temperature for 10 minutes, then heated to reflux for 1 hour. Then cooled to room temperature, the solvent and volatiles were removed under vacuum to give 781 mg of crude product D, (R)-1-chloro-N,N-dimethylpropan-2-amine HCl (yield Yield 93%), which was used directly in the following steps.
[0321] KOtBu (617 mg, 5.5 mmol) was added to a stirred solution of the above (R)-1-chloro-N,N-dimethylpropan-2-amine HCl, intermediate D, in DMF (5.0 mL) under Ar. The resulting mixture was stirred at room temperature for 10 minutes. Diphenylacetonitrile Ph in DMF (5.0 mL) 2 In a separate flask ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com