Medicinal uses of the ubiquitin protease usp7 in aging and related diseases
A technology of protease and aging, which is applied in the preparation of drugs for the prevention and treatment of aging-related diseases, and the medical application field of ubiquitin protease USP7 in aging and related diseases, which can solve problems such as telomere damage, telomere shortening, and cell aging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
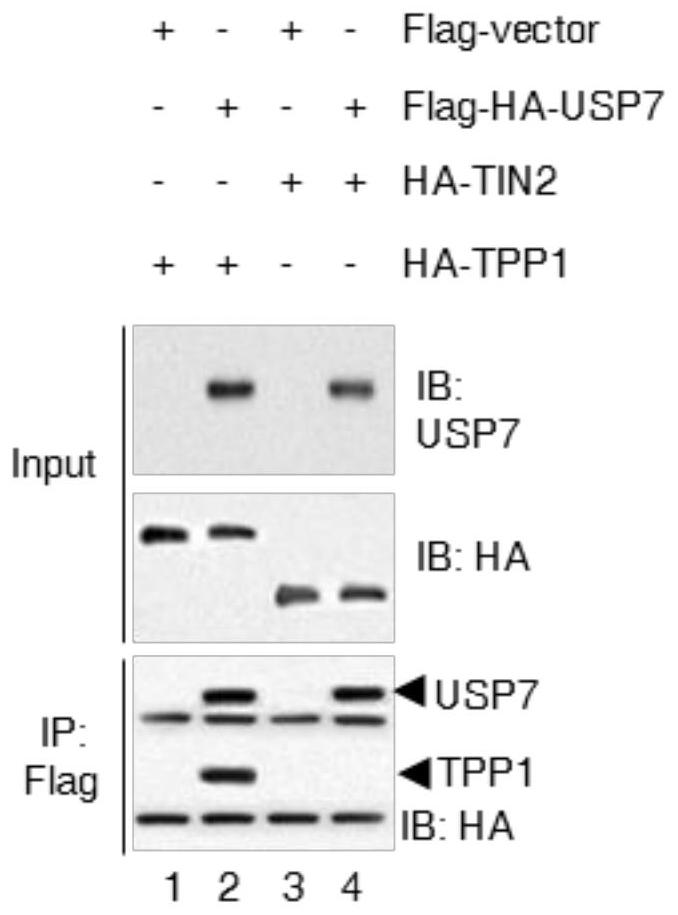
[0017] Example 1 USP7 binds to TPP1 at the telomere
[0018] Experimental objects: HT1080 cells, 293T cells and Hela cells.
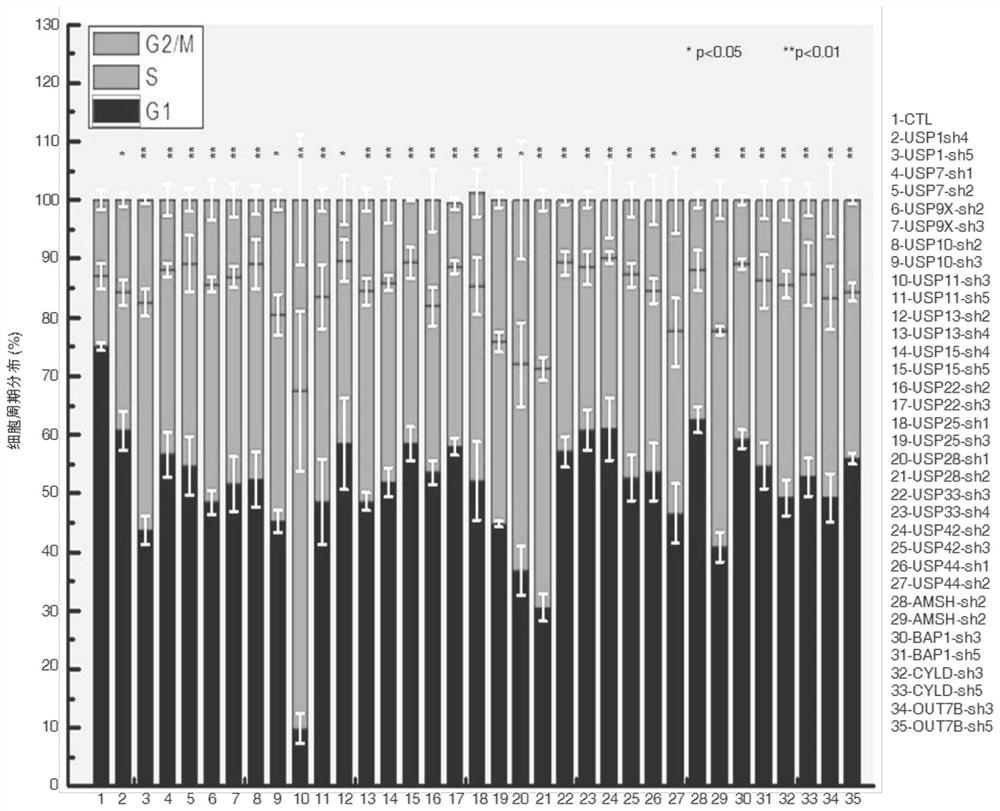
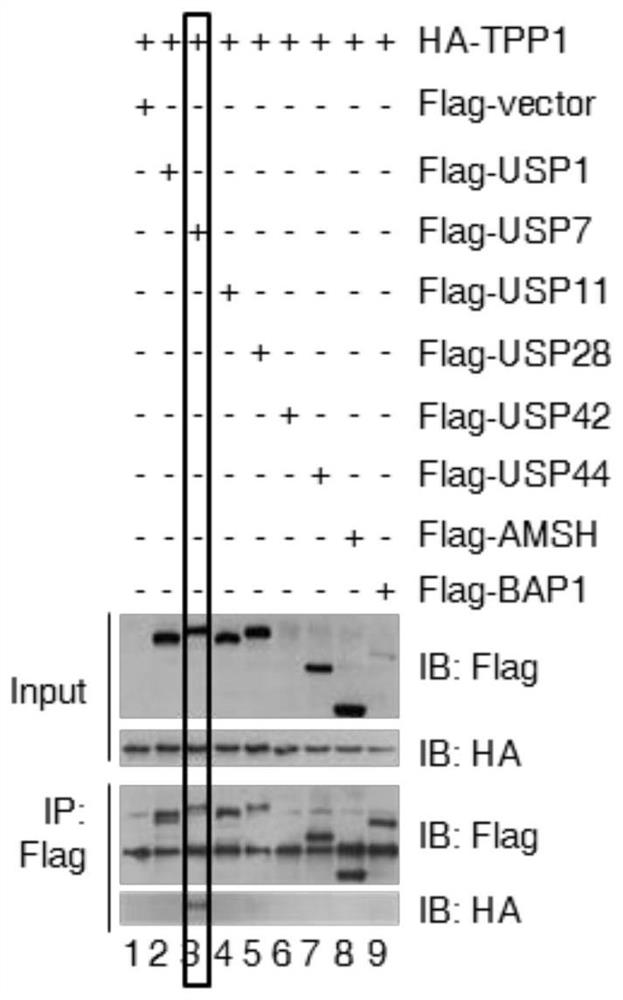
[0019] Experimental method: HT1080 cells with stable and low expression of 17 deubiquitinases were constructed using lentivirus, and the cell cycle distribution was detected by flow cytometry; 293T cells were transfected with HA-tagged TPP1 and Flag-tagged deubiquitinases, transfected After 36 hours of transfection, the combination of deubiquitinase and TPP1 was detected by co-immunoprecipitation; the binding of deubiquitinase and telomeric DNA was detected by chromatin immunoprecipitation; USP7 was detected by immunofluorescence and FISH in Hela cells Binding to telomeres and TPP1.
[0020] The results showed that: among the 17 deubiquitinating enzymes screened ( Figure 1A ), USP7 specifically binds to TPP1 and telomeric DNA ( Figure 1B -D). Although only 20% of USP7 co-localized with telomeres, more than 90% of USP7 co-localized with TPP1 ( Fig...
Embodiment 2
[0021] Example 2 USP7 regulates TPP1 ubiquitination degradation and cell senescence
[0022] Experimental objects: HT1080 cells and Hela cells with stable and low expression of USP7.
[0023]Experimental method: Use lentivirus to construct HT1080 cells with stable and low expression of USP7, and detect the expression of TPP1 by immunofluorescence; use flow cytometry to detect cell cycle distribution and -gal staining to detect cell senescence; HT1080 cells are irradiated with X-rays, and real-time Quantitative PCR and western blotting to detect the mRNA and protein expression of FBW7, TPP1 and USP7; Hela cells were transfected with siRNA against FBW7 and USP7 to inhibit their expression, real-time quantitative PCR was used to detect the interference efficiency of USP7 and FBW7; immunofluorescence was used to detect TPP1 The expression of TPP1 and HA-tagged ubiquitin and siRNA of FBW7 or USP7 were co-transfected in 293T cells, and the ubiquitination of TPP1 was detected by co-i...
Embodiment 3
[0025] Example 3 USP7 overcomes stress-induced lung aging and fibrosis
[0026] Experimental subjects: 12 wild-type mice aged 3-6 months.
[0027] Experimental method: Take 12 wild-type mice, divide them into 4 groups, 3 mice in each group, and carry out the following treatments respectively, the trachea is infused with control or USP7 lentivirus, and the trachea is infused with PBS or bleomycin. The specific method: perfusion on the first day The control or USP7 lentivirus was perfused with PBS or bleomycin on the second day, and the control or USP7 lentivirus was perfused every 4 days thereafter. On the 22nd day, the mice were intubated to detect the respiratory function. Extract lung tissue RNA, detect the mRNA expression of USP7, TPP1, HP1g, SPC, -SMA, T1, Col6a and s100a4 by real-time quantitative PCR; lung tissue paraffin-embedded sections, Masson and H&E staining, use immunofluorescence to detect lung type II cells Percentage (number of SPC positive cells) and -SMA exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com