Method for preparing methyl furoate from furfuraldehyde through oxidation and esterification
A technology of methyl furoate and oxidative esterification, which is applied in the direction of organic chemistry, can solve the problems of increased cost, inability to recycle, difficult separation of homogeneous alkali, etc., and achieve the effect of reducing loss and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
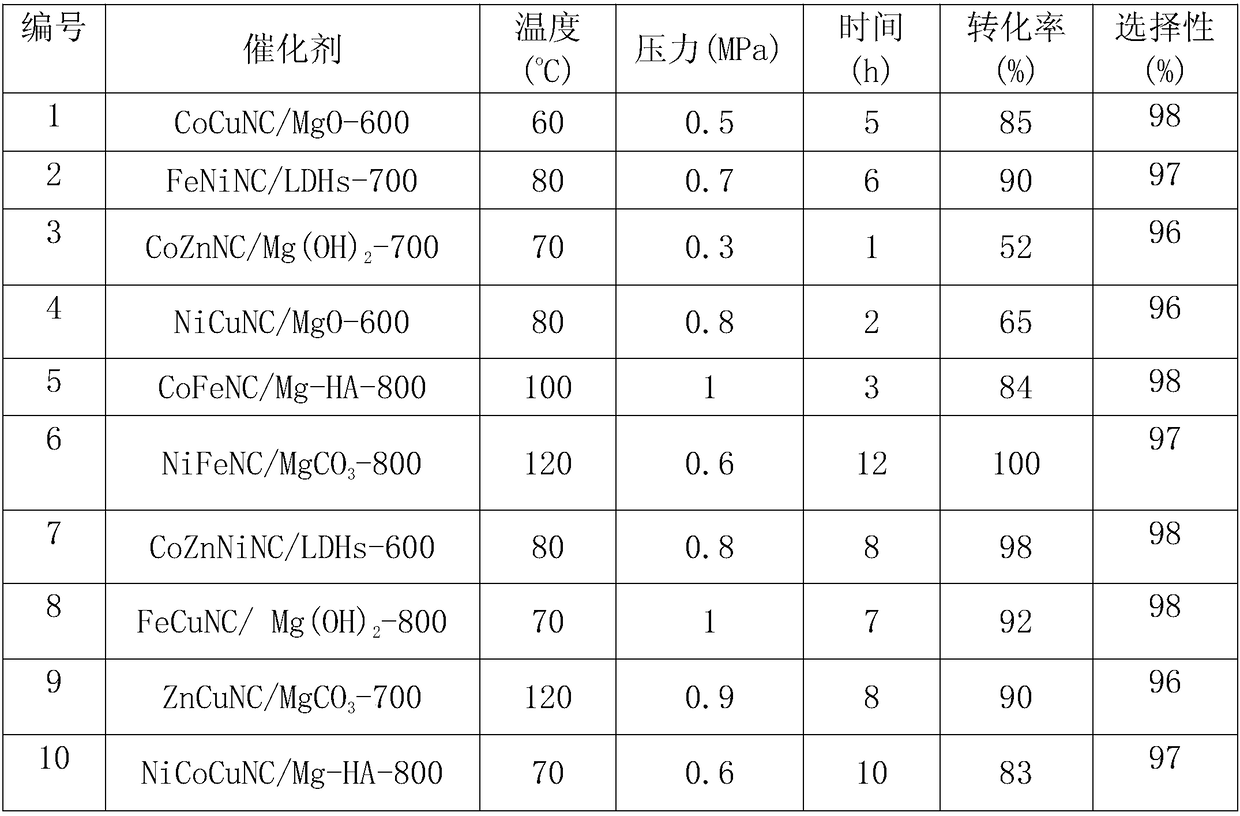
[0012] Add CoCuNC / MgO-600 (Co 3.7wt%, Cu 1.5wt%, N 1%, C 12%) catalyst, 0.5mmol furfural, and 5mL methanol into a stainless steel autoclave with polytetrafluoroethylene lining inside. The temperature was raised to a reaction temperature of 60° C. using an automatic temperature controller, and 0.5 MPa of oxygen was added to react for 5 hours. During the reaction, the pressure was kept constant. The reaction products were analyzed by GC, and the reaction results are shown in Table 1.
Embodiment 2
[0014] FeNiNC / LDHs-700 (Fe 2.6wt%, Ni 2wt%, N 3%, C 15%) catalyst, 0.5mmol furfural, and 5mL methanol were added to a stainless steel autoclave with a polytetrafluoroethylene lining inside. The temperature was raised to a reaction temperature of 80° C. using an automatic temperature controller, and 0.6 MPa oxygen was added to react for 7 hours, keeping the pressure constant during the reaction. The reaction product was analyzed by GC, and the reaction results are shown in Table 1
Embodiment 3
[0016] CoZnNC / Mg(OH) 2 -700 (Co 5wt%, Zn 1.5wt%, N 0.5%, C 10%) catalyst, 0.5mmol furfural, and 5mL methanol were added to a stainless steel autoclave with a polytetrafluoroethylene lining inside. The temperature was raised to a reaction temperature of 70° C. using an automatic temperature controller, and 0.3 MPa oxygen was added to react for 1 hour. During the reaction, the pressure was kept constant. The reaction products were analyzed by GC, and the reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com