Diagnosis kit for rectal carcinoma prognosis early warning and application of diagnosis kit
A colorectal cancer and kit technology, applied in the biological field, can solve problems such as difficulty in eliminating the interference of tumor heterogeneity, inability to obtain tumor diagnosis, staging, and classification standards, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1. Acquisition of immune-related genes related to the prognosis of colorectal cancer
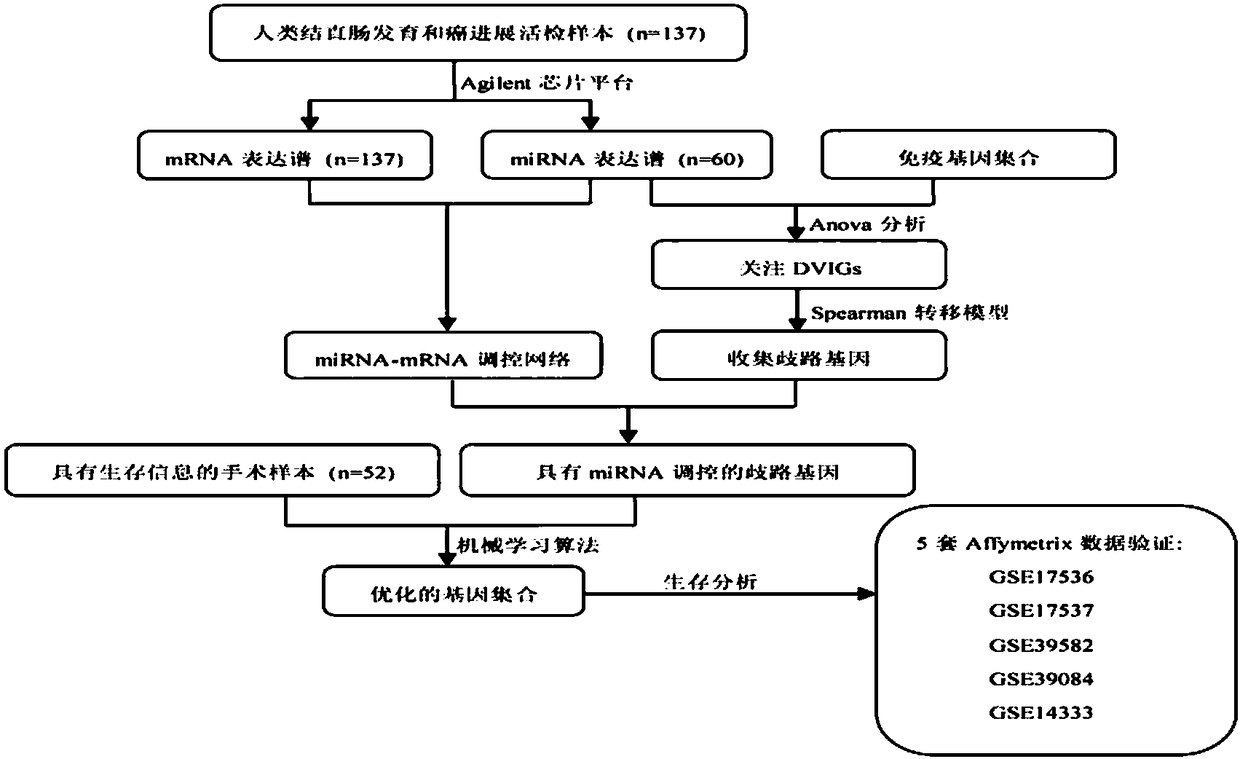
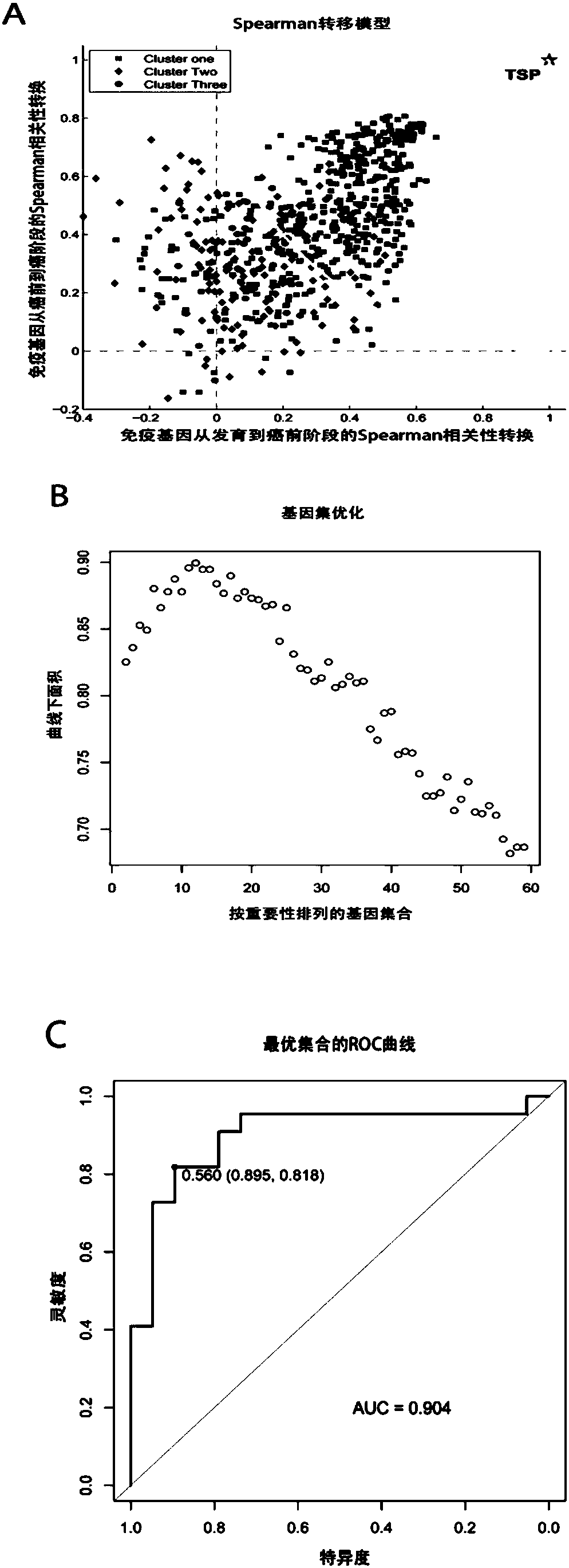
[0049] In this example, the Spearman metastasis model was used to discover the immune-related genes AXL (sequence 1), BCL3 (sequence 2), ABR (sequence 3), MAP3K1 (sequence 4), CASP8 (sequence 5), RPS6KA1 ( Sequence 6), VASP (sequence 7), SIRT1 (sequence 8), BCL2L1 (sequence 9), AKIRIN2 (sequence 10), PIN1 (sequence 11), MEF2A (sequence 12) and PCBP2 (sequence 13), using the Spearman transfer model A bioinformatics pipeline for the discovery of immune-related genes associated with colorectal cancer prognosis figure 1 shown. Specific steps are as follows:
[0050] 1. Experimental materials
[0051] 1. Human colorectal embryonic development tissue specimens and classification
[0052] The 20 cases of human colorectal embryo development samples in the present invention were taken from the artificial abortions handled by the Maternal and Child Health Hospital of Haidian Distric...
Embodiment 2
[0097] Example 2. Application of immune-related genes in the prognosis of patients with colorectal cancer
[0098] 1. Test sample
[0099] The test samples included 10 colorectal cancer surgical resection samples with overall survival information and 2 normal adult colorectal mucosa samples. The collected clinical samples were taken from the clinical samples of Zhejiang University School of Medicine, all clinical data were complete, and the sample collection process was standardized, and no obvious RNA degradation occurred (the RNA integrity was identified by the Agilent 2100 Bioanalyzer system and the score was greater than or equal to 5 points). All the above samples obtained the informed consent of the patients, and the samples combined with genetic diseases or other diseases such as diabetes or autoimmune diseases were excluded. The postoperative survival time of 10 samples were patient 1 (7.32 years), patient 2 (7.15 years), patient 3 (6.82 years), patient 4 (7.23 years)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com