A kind of immunoregulatory mixture and its application
A technology of immune regulation and mixture, which is applied in the direction of drug combination, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of high toxicity, side effects, difficulties, unfavorable saving of blood sources and medical expenses of immunosuppressants, and achieve saving Blood source, less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
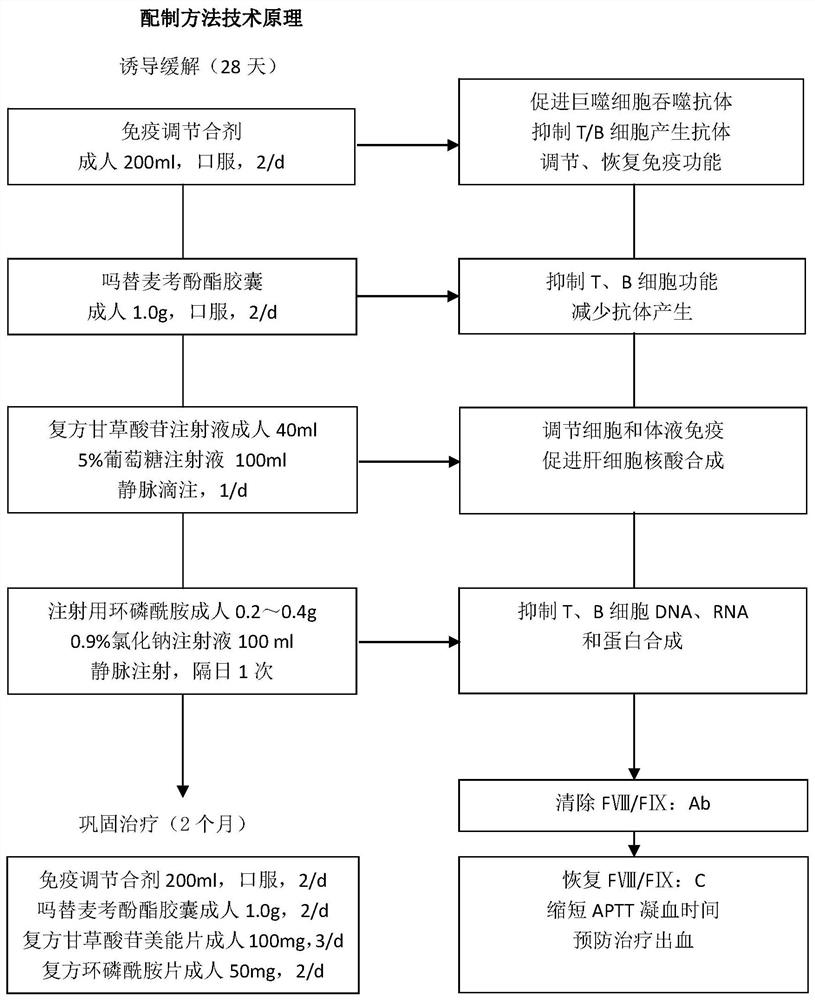
[0034] This example provides an immunomodulatory mixture, which is prepared by decocting 200ml of the following medicines: Radix Astragali: 30g, Ginseng: 10g, Atractylodes Rhizoma: 15g, Turtle Shell: 15g, Ligustrum lucidum: 30g, Angelica: 12g, Paeoniae Rubra : 12g, paeonol: 12g, raw rehmannia: 15g, flavescens: 6g, rhubarb: 10g, roasted licorice: 6g.
[0035]The preparation method of the above-mentioned immunomodulatory mixture is to soak the medicine in the formula with an appropriate amount of water for more than 2 hours, then boil it on a high fire and then change it to a low heat and boil it for 1 hour. After the medicine liquid is concentrated to 200ml, pour it out, and then add an appropriate amount of water and repeat the boil. Once, pour out the medicinal solution after it is concentrated to 200ml, discard the medicinal residues, combine and mix the medicinal solutions twice, divide into 2 bags, each bag is 200ml.
[0036] Brief Introduction of Drugs in Immunomodulatory...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com