Polyacrylate solid polymer electrolyte and preparation method thereof and application thereof in solid lithium battery
A polyacrylate, solid polymer technology, applied in the field of solid polymer electrolytes, can solve the problems of poor independent film-forming performance, difficulty in large-scale preparation of solid electrolytes, and inability to guarantee safety performance, and achieves improved mechanical strength, shortened The effect of simple film production time and production method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
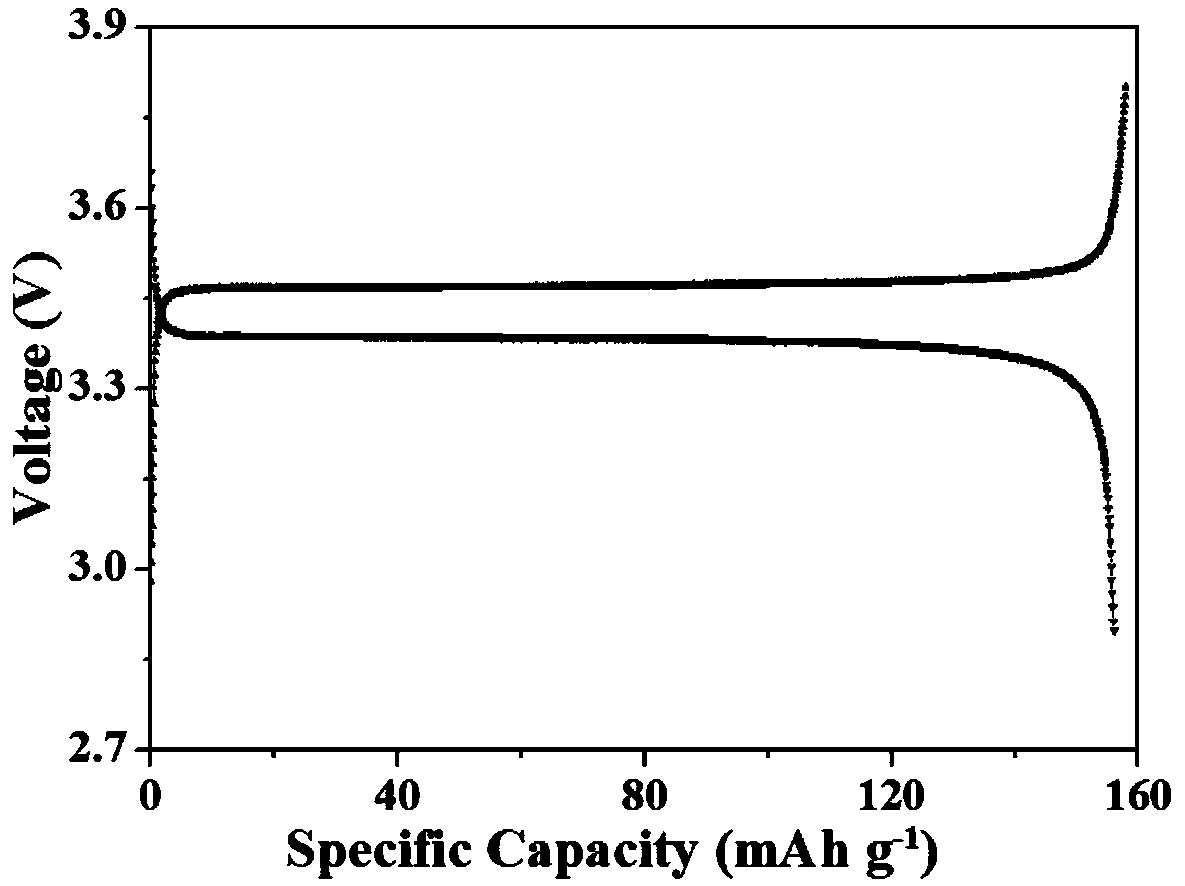
Embodiment 1
[0026] The present invention is a polyacrylate solid polymer electrolyte, the solid polymer electrolyte includes acrylate, lithium salt, and initiator, and the acrylate accounts for 60% of the solid polymer electrolyte. The proportion of lithium salt in this solid polymer electrolyte is 40%.
[0027] The acrylate is butyl methacrylate.
[0028] The lithium salt comprises lithium perchlorate.
[0029] The initiator is azobisisobutyronitrile, and the initiator accounts for 0.3% of the total mass of the acrylate and the crosslinking agent.
[0030] A method for preparing a polyacrylate solid polymer electrolyte, step 1: mix butyl methacrylate and lithium perchlorate into a 20ml reagent bottle, stir at room temperature for 30min, so that butyl methacrylate and perchlorate Lithium acid mixed evenly;
[0031] Step 2: Add azobisisobutyronitrile to the above mixture, stir at room temperature for 5 minutes, and mix well;
[0032] Step 3: Evenly pour the mixed solution obtained abov...
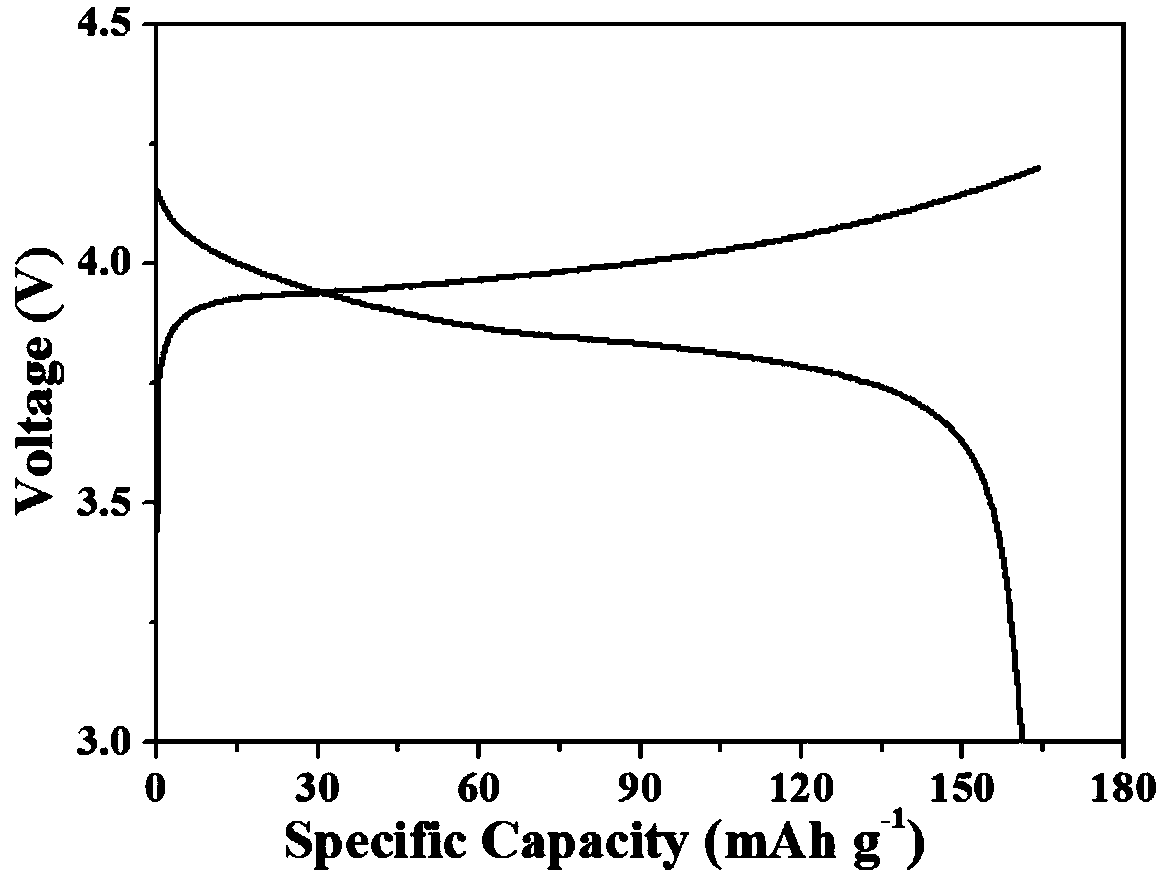
Embodiment 2
[0036] In order to obtain an all-solid polymer electrolyte with high mechanical strength, the solid polymer electrolyte includes acrylate, lithium salt, initiator and crosslinking agent, and the acrylate accounts for 40% of the solid polymer electrolyte, so The proportion of the lithium salt in the solid polymer electrolyte is 40%, and the proportion of the crosslinking agent in the solid polymer electrolyte is 20%.
[0037] The acrylate is methyl methacrylate.
[0038] The lithium salt comprises lithium triflate.
[0039] The initiator is azobisisoheptanonitrile, and the initiator accounts for 0.5% of the total mass of the acrylate and the crosslinking agent.
[0040] Described cross-linking agent is polyethylene glycol diacrylate, methacryloxypropyl trimethoxysilane, styrene, tetraethylene glycol dimethacrylate, glycidyl methacrylate One or more, preferably polyethylene glycol diacrylate.
[0041] A method for preparing a polyacrylate solid polymer electrolyte, step 1: mi...
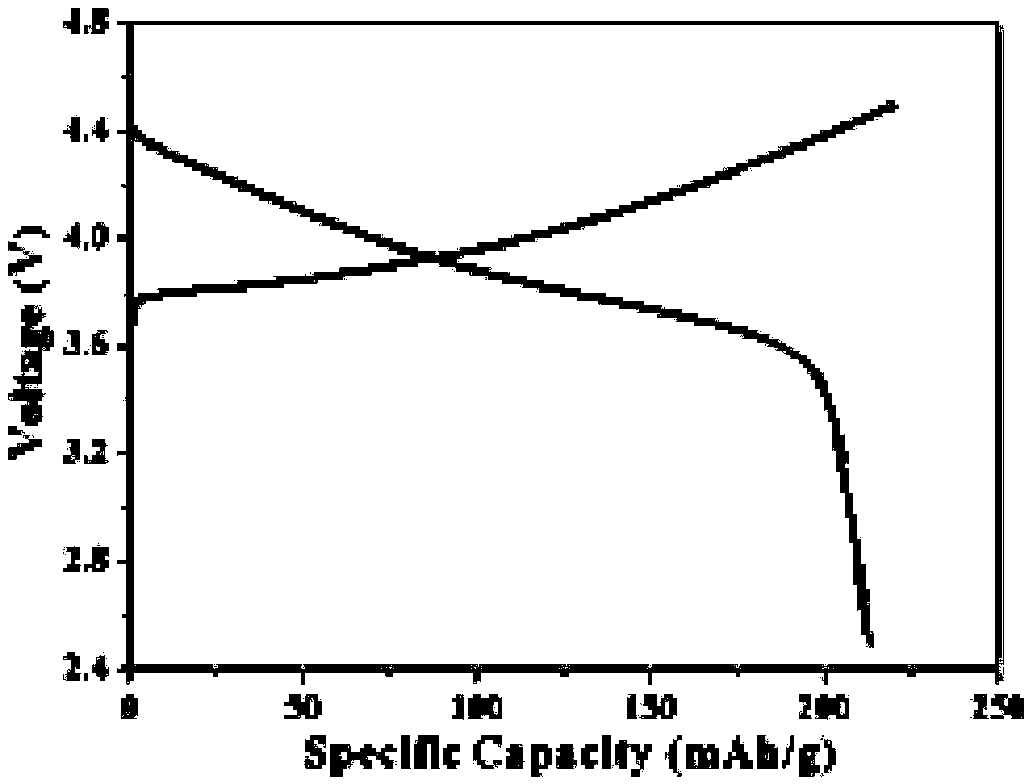
Embodiment 3
[0048] In order to further improve the mechanical strength of the all-solid polymer electrolyte, this solid polymer electrolyte includes acrylate, lithium salt, initiator, crosslinking agent and porous rigid support material, and the proportion of the acrylate in this solid polymer electrolyte is The ratio is 40%, the proportion of the lithium salt in the solid polymer electrolyte is 40%, and the proportion of the crosslinking agent in the solid polymer electrolyte is 20%.
[0049] The acrylate is ethyl methacrylate.
[0050] The lithium salt comprises lithium bis(trifluoromethanesulfonate)imide.
[0051]The initiator is dibenzoyl peroxide, and the initiator accounts for 0.7% of the total mass of the acrylate and the crosslinking agent.
[0052] Described cross-linking agent is polyethylene glycol diacrylate, methacryloxypropyl trimethoxysilane, styrene, tetraethylene glycol dimethacrylate, glycidyl methacrylate One or more, preferably styrene.
[0053] The porous rigid sup...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com