Cells expressing TH1 characteristics and cytolytic properties
A cell activity and cell technology, applied in the direction of cell culture active agents, animal cells, vertebrate cells, etc., can solve the problems of recognizing epitopes, lack of animal models, affecting characteristics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
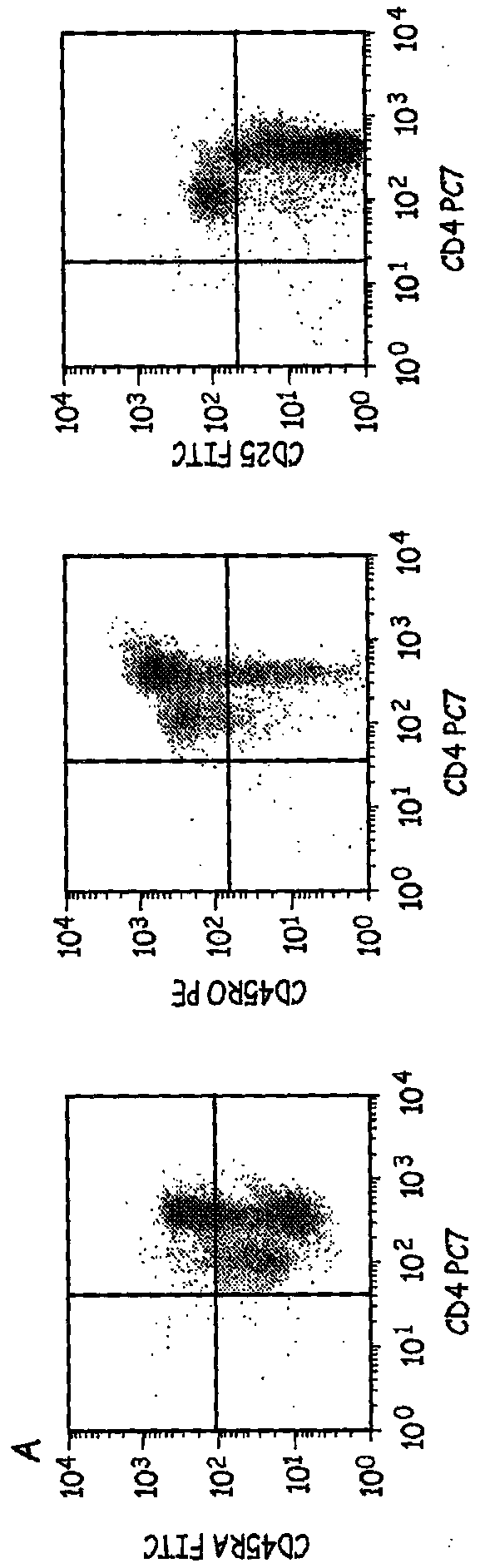
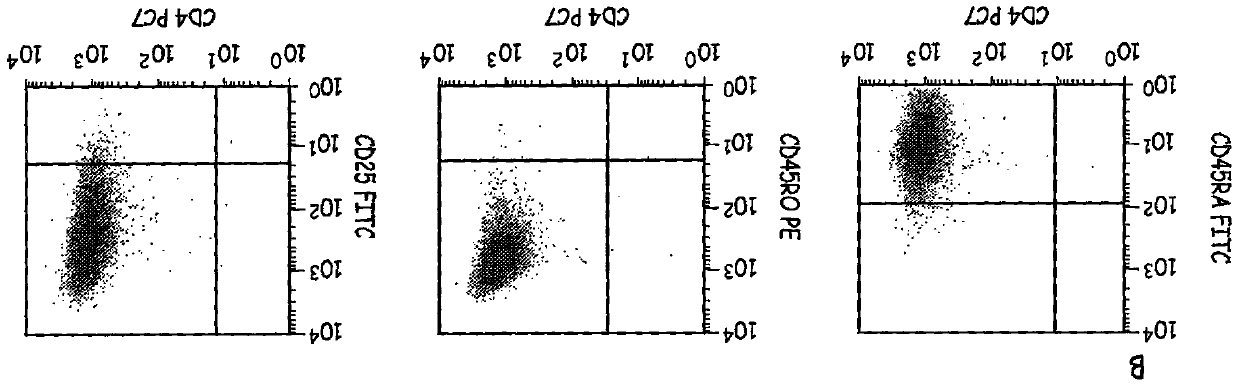
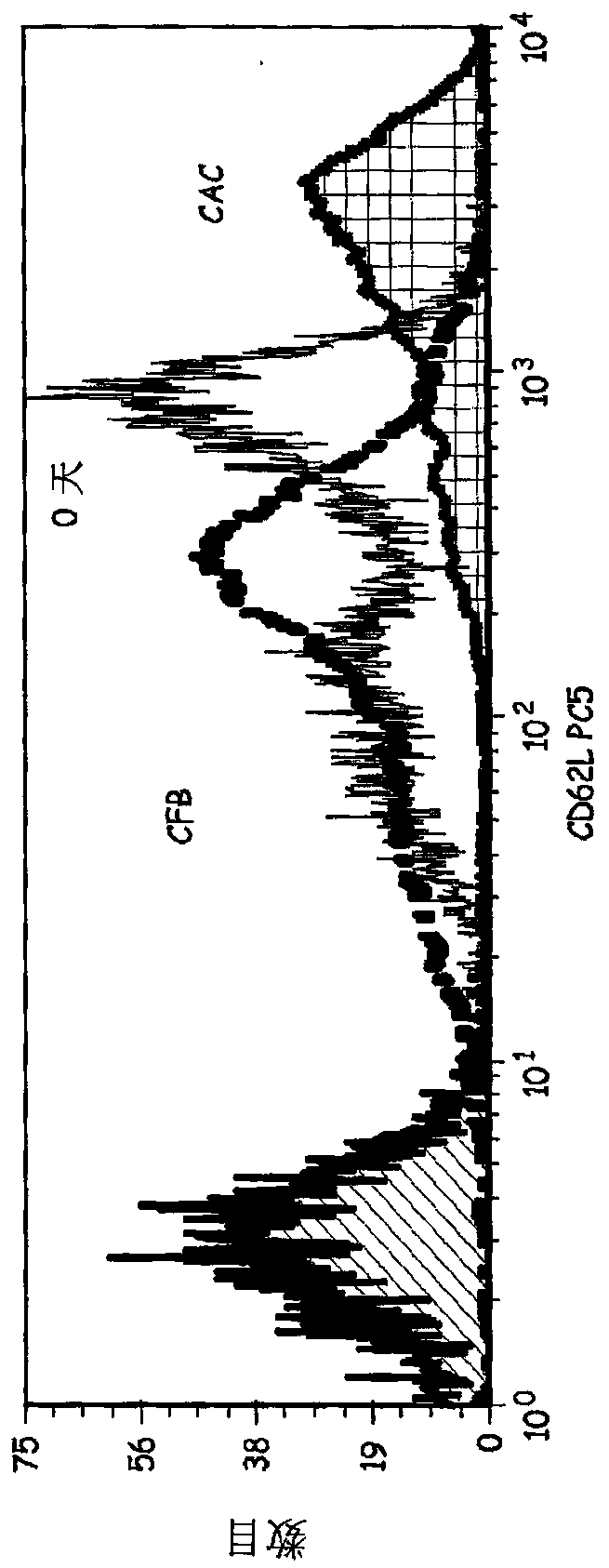
[0059] Preparation of activated cells in culture (CAC)—CD4+ T-cells were cultured for 9 days in the presence of CD3 / CD28 ClinEx Vivo Dynal beads obtained from Invitrogen. (Location). Cells were grown in Life Cell Culture Flasks (Baxter) at 37°C and 5% CO 2 were grown and restimulated with beads on days 3 and 6 of culture. After 9 days in culture, the beads can be removed and these cells are termed CACs.
[0060] Preparation of cells in formulation buffer (CFB) - CACs were placed into cRPMI medium for washing. The time is recorded to indicate the start of the formulation protocol. Cells in cRPMI were centrifuged, supernatant removed, and cells resuspended in cRPMI buffer. Cell viability was determined by using the trypan blue assay. The percentage of viable cells was determined using the total cell number and concentration of viable cells. If the sample has greater than 80% cell viability, the steps continue for reactivation and formulation of the cells.
[0061] CAC cel...
example 2
[0077] Example 2 - Direct killing by activated Th1 / killer cells. To test whether CFB could have a direct effect on tumor cells, the cells were tested for their ability to kill the ARH77 cell line. This cell line is an established myeloma cell line. ARH77 cells were stained in CFSE to distinguish ARH77 cells from CFB. CFB was mixed with stained ARH77 cells for 18 hours with different effector (CFB) and target (ARH77). After this time, 7AAD was added and cells were acquired using a FC500MPL flow cytometer. The experiment was repeated on different batches of CAC as well as on fresh and thawed CFB. ARH77 alone, ARH77 with DynaBeads, CD4+ cells or ARH77 with CAC had no significant death (less than 10%) data, not shown. Figure 5 The results shown demonstrate that CFB, but not CAC, CD4+ cells or beads, has a direct killing effect on the ARH77 cell line.
[0078] The nature of the direct killing effect was examined to see if the effect was due to the perforin-granzyme pathway....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com