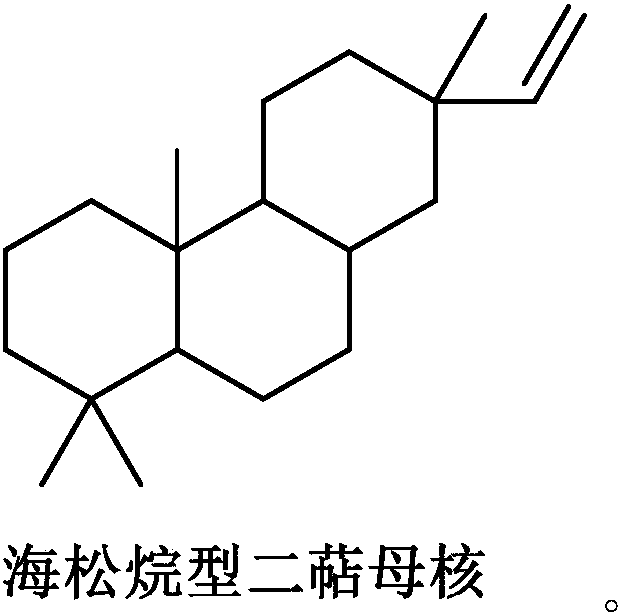
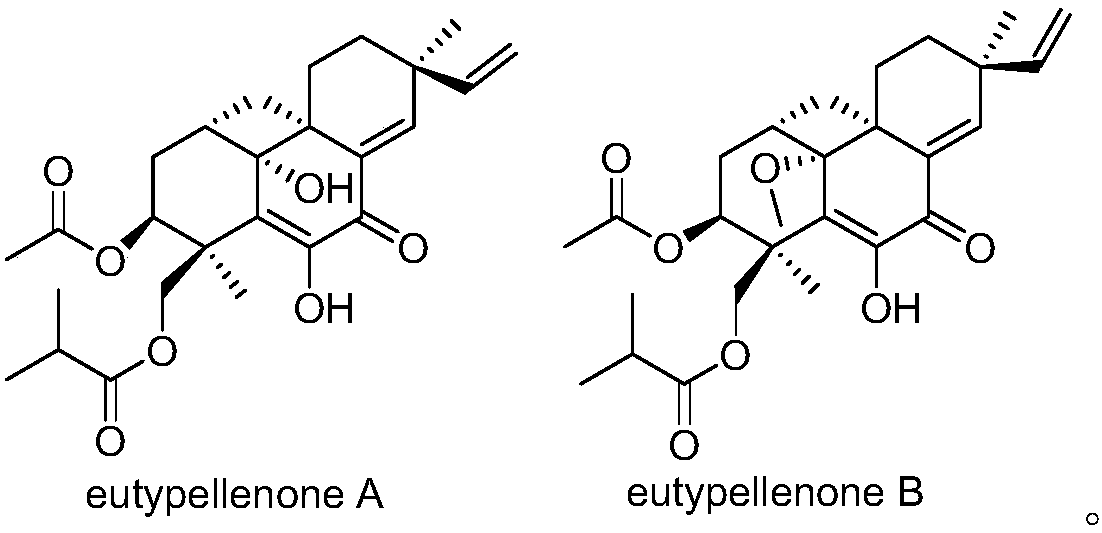
Pimarane diterpene compounds containing four-membered rings and antitumor application thereof
A kind of technology of pimamarane diterpenes and compounds, which is applied in the preparation of antitumor drugs, the field of pimamarane diterpenoids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1. Preparation of compounds of the present invention
[0038] The strain used in the present invention is isolated from Arctic Curvularia sp.D-1 (Eutypella sp.D-1) from the soil sample of London Island (100 meters above sea level) in Kongsfjorden, was preserved in the China Center for Type Culture Collection (CCTCC) on April 12, 2013. The deposit number is CCTCC NO: M 2013144.
[0039] 1. Prepare the total crude extract
[0040] 1) pick a small amount of mycelium of the strain of Eutypella sp. D-1 whose preservation number is CCTCC NO: M 2013144 from the plate, inoculate it into a 250mL Erlenmeyer flask for seed culture, and each Erlenmeyer flask is equipped with 100mL of seed culture medium, Cultivate at a constant temperature on a shaker at 28°C, with a rotation speed of 180r / min, and cultivate for 3.5 days to obtain a first-class seed liquid, insert the same seed medium according to the inoculation amount of 5% (v / v), and cultivate at 28°C, 180r / min on ...
Embodiment 2
[0059] Embodiment 2 The in vitro antitumor activity experiment of the compound of the present invention:
[0060] Method: CCK8 method (references: Tominaga H, Ishiyama M, Ohseto F, et al.A water-soluble tetrazolium salt useful for colorimetric cell viability assay[J].Analytical Communications,1999,36(2):47-50. )
[0061] Human cervical cancer cells Hela, human breast cancer cells MCF-7, human colon cancer cells HCT-116, human chronic leukemia cells K562, and human pancreatic cancer cells SW1990 in the logarithmic growth phase were respectively taken at a cell density of 5000 cells / well. Inoculated in 96-well plates, placed at 37 °C 5% CO 2 cultured in a constant temperature incubator. After 24 hours, add 10 μL of samples of different concentrations to the 96-well plate, so that the final volume of the solution in the plate is 100 μL. After continuing to cultivate in the incubator for 48 hours, add 10 μL of CCK-8 solution and continue to incubate for about 1 hour. Take it out...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com